Project-1: STING-seq
Last updated: 2022-08-25
Checks: 7 0
Knit directory: rotation2/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20220607) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 4d8f9d5. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Unstaged changes:
Modified: .RData
Modified: .Rhistory
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/project_1.Rmd) and HTML
(docs/project_1.html) files. If you’ve configured a remote
Git repository (see ?wflow_git_remote), click on the
hyperlinks in the table below to view the files as they were in that
past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| html | 268c781 | chenh19 | 2022-08-25 | Build site. |
| html | d5675d8 | chenh19 | 2022-08-25 | Build site. |
| html | 6f9b2d0 | chenh19 | 2022-08-25 | Build site. |
| html | eb7f6e2 | chenh19 | 2022-08-25 | Build site. |
| html | 06d8f5b | chenh19 | 2022-08-25 | Build site. |
| html | 481c95e | chenh19 | 2022-08-25 | Build site. |
| html | 4c40591 | chenh19 | 2022-08-25 | update |
| html | 210ce87 | chenh19 | 2022-08-25 | Build site. |
| html | 7126807 | chenh19 | 2022-08-25 | Build site. |
| html | e576eee | chenh19 | 2022-08-25 | Build site. |
| html | 3b152ba | chenh19 | 2022-08-25 | Build site. |
| html | abd8577 | chenh19 | 2022-08-25 | Build site. |
| html | fb0804c | chenh19 | 2022-08-25 | Build site. |
| html | 46c0eee | chenh19 | 2022-08-25 | Build site. |
| html | 4bcd287 | Hang Chen | 2022-08-11 | Build site. |
| html | 316e143 | Hang Chen | 2022-08-11 | Build site. |
| html | 2ecff7a | Hang Chen | 2022-08-11 | Build site. |
| html | 1a7329a | Hang Chen | 2022-08-11 | Build site. |
| html | d98b8ed | Hang Chen | 2022-08-10 | Build site. |
| html | afbc7f7 | chenh19 | 2022-08-09 | Build site. |
| html | f3ddb60 | chenh19 | 2022-08-09 | Build site. |
| html | aeeaff4 | chenh19 | 2022-08-09 | Build site. |
| html | faa4371 | chenh19 | 2022-08-09 | Build site. |
| html | 40d7285 | chenh19 | 2022-08-09 | Build site. |
| html | 51ff922 | chenh19 | 2022-08-09 | Build site. |
| html | 60bb670 | chenh19 | 2022-08-09 | Build site. |
| html | 5035de6 | chenh19 | 2022-08-09 | Build site. |
| html | c710b98 | chenh19 | 2022-08-09 | Build site. |
| html | 9c8a125 | chenh19 | 2022-08-09 | Build site. |
| html | 759a524 | chenh19 | 2022-08-09 | Build site. |
| html | 0137730 | chenh19 | 2022-08-09 | Build site. |
| html | 306629d | chenh19 | 2022-08-09 | Build site. |
| html | a2f14fc | chenh19 | 2022-08-09 | Build site. |
| html | afe9e5e | chenh19 | 2022-08-09 | Build site. |
| html | 7f87abe | chenh19 | 2022-08-09 | Build site. |
| html | f7a30e6 | chenh19 | 2022-08-08 | Build site. |
| html | ae6dc22 | chenh19 | 2022-08-08 | Build site. |
| html | 31345e3 | chenh19 | 2022-08-08 | Build site. |
| Rmd | 14daeb5 | chenh19 | 2022-08-08 | wflow_publish("./analysis/*.Rmd") |
| html | ab573d7 | chenh19 | 2022-08-08 | Build site. |
| html | 82d0b8a | chenh19 | 2022-08-08 | Build site. |
| html | 63022aa | chenh19 | 2022-08-08 | Build site. |
| html | b4ec414 | chenh19 | 2022-08-08 | Build site. |
| html | fb4fa31 | chenh19 | 2022-08-08 | Build site. |
| html | 03df33f | chenh19 | 2022-08-08 | Build site. |
| html | ec5763a | chenh19 | 2022-08-08 | Build site. |
| Rmd | d6b5331 | chenh19 | 2022-08-08 | wflow_publish("./analysis/*.Rmd") |
| html | 870ec95 | chenh19 | 2022-08-08 | Build site. |
| Rmd | 26c6105 | chenh19 | 2022-08-08 | wflow_publish("./analysis/*.Rmd") |
| html | 4df0f61 | chenh19 | 2022-08-08 | Build site. |
| html | 8bad269 | chenh19 | 2022-08-08 | Build site. |
| Rmd | e769869 | chenh19 | 2022-08-08 | wflow_publish("./analysis/*.Rmd") |
| html | 6e10041 | chenh19 | 2022-08-08 | Build site. |
| Rmd | e57b4ef | chenh19 | 2022-08-08 | wflow_publish("./analysis/*.Rmd") |
| html | b045b81 | chenh19 | 2022-08-08 | Build site. |
| Rmd | 26a8d3b | chenh19 | 2022-08-08 | wflow_publish("./analysis/*.Rmd") |
| html | e22ab6b | chenh19 | 2022-08-08 | Build site. |
| Rmd | b57b687 | chenh19 | 2022-08-08 | wflow_publish("./analysis/*.Rmd") |
| html | 87b3f9d | chenh19 | 2022-08-08 | Build site. |
| Rmd | 9a06a06 | chenh19 | 2022-08-08 | update |
| html | 78b6bd6 | chenh19 | 2022-08-08 | Build site. |
| html | 60fabb8 | Hang Chen | 2022-08-08 | Build site. |
| html | cee42b8 | Hang Chen | 2022-08-05 | Build site. |
| html | 6927e45 | Hang Chen | 2022-08-04 | Build site. |
| html | 551a34f | Hang Chen | 2022-08-04 | Build site. |
| html | 80908a7 | Hang Chen | 2022-08-04 | Build site. |
| html | 2623d6b | Hang Chen | 2022-08-04 | Build site. |
| html | e9d9966 | Hang Chen | 2022-08-04 | Build site. |
| html | 57d96a8 | Hang Chen | 2022-08-04 | update |
| Rmd | 05b3310 | Hang Chen | 2022-08-04 | update |
| html | 05b3310 | Hang Chen | 2022-08-04 | update |
| html | 37d15c9 | chenh19 | 2022-07-19 | Build site. |
| html | 8f6816e | chenh19 | 2022-07-19 | Build site. |
| html | 4a94b94 | chenh19 | 2022-07-19 | Build site. |
| Rmd | a18fc1f | chenh19 | 2022-07-19 | wflow_publish("./analysis/*.Rmd") |
| html | 870115f | chenh19 | 2022-07-19 | Build site. |
| Rmd | 6307f5c | chenh19 | 2022-07-19 | wflow_publish("./analysis/*.Rmd") |
| html | 9241fe6 | chenh19 | 2022-07-08 | Build site. |
| Rmd | abd8a0c | chenh19 | 2022-07-08 | wflow_publish("./analysis/*.Rmd") |
| html | 0a7633b | chenh19 | 2022-07-07 | Build site. |
| Rmd | 06d53d1 | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| html | 63535ad | chenh19 | 2022-07-07 | Build site. |
| Rmd | fe2a82b | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| Rmd | 9898acd | chenh19 | 2022-07-07 | update |
| html | feb5923 | chenh19 | 2022-07-07 | Build site. |
| Rmd | 9eae283 | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| html | c849244 | chenh19 | 2022-07-07 | Build site. |
| Rmd | 29eb161 | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| html | 7ab5d71 | chenh19 | 2022-07-07 | Build site. |
| Rmd | 2baffc8 | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| html | 625e7ca | chenh19 | 2022-07-07 | Build site. |
| Rmd | bb3e1aa | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| html | 0e1d92d | chenh19 | 2022-07-07 | Build site. |
| Rmd | 138b4fe | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| html | 24d6bcf | chenh19 | 2022-07-07 | Build site. |
| Rmd | 941e24b | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| html | 3a14766 | chenh19 | 2022-07-07 | Build site. |
| Rmd | d425ac9 | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| html | ca77db2 | chenh19 | 2022-07-07 | Build site. |
| Rmd | 608cdf8 | chenh19 | 2022-07-07 | wflow_publish("./analysis/*.Rmd") |
| Rmd | c639389 | chenh19 | 2022-07-06 | update |
| html | e0cb7a2 | chenh19 | 2022-06-28 | Build site. |
| Rmd | ac33ff0 | chenh19 | 2022-06-28 | wflow_publish("./analysis/*.Rmd") |
| html | bf05c98 | chenh19 | 2022-06-28 | Build site. |
| Rmd | 1c8bb9a | chenh19 | 2022-06-28 | wflow_publish("./analysis/*.Rmd") |
| html | 7cfb685 | chenh19 | 2022-06-28 | Build site. |
| Rmd | 82fce8f | chenh19 | 2022-06-28 | wflow_publish("./analysis/*.Rmd") |
| html | 43dad9c | chenh19 | 2022-06-28 | Build site. |
| Rmd | 20718e9 | chenh19 | 2022-06-28 | wflow_publish("./analysis/*.Rmd") |
| html | 7aa3172 | chenh19 | 2022-06-28 | Build site. |
| Rmd | e0425d8 | chenh19 | 2022-06-28 | wflow_publish("./analysis/*.Rmd") |
| html | 2254608 | chenh19 | 2022-06-28 | Build site. |
| Rmd | 754150e | chenh19 | 2022-06-28 | wflow_publish("./analysis/*.Rmd") |
| html | 968a4b6 | chenh19 | 2022-06-23 | Build site. |
| html | 229f924 | chenh19 | 2022-06-23 | Build site. |
| Rmd | c7847bd | chenh19 | 2022-06-23 | wflow_publish("./analysis/*.Rmd") |
| html | 9750134 | chenh19 | 2022-06-23 | Build site. |
| Rmd | 0064ff3 | chenh19 | 2022-06-23 | wflow_publish("./analysis/*.Rmd") |
| html | 4b38bf6 | chenh19 | 2022-06-23 | Build site. |
| Rmd | 4eb11c9 | chenh19 | 2022-06-23 | wflow_publish("./analysis/*.Rmd") |
| html | 8a7980d | chenh19 | 2022-06-23 | Build site. |
| html | 3e1478e | chenh19 | 2022-06-22 | Build site. |
| html | 5258612 | chenh19 | 2022-06-22 | Build site. |
| Rmd | ecfd58d | chenh19 | 2022-06-22 | wflow_publish("./analysis/*.Rmd") |
| html | 2382868 | chenh19 | 2022-06-22 | Build site. |
| Rmd | 694470f | chenh19 | 2022-06-22 | wflow_publish("./analysis/*.Rmd") |
| html | 1144127 | chenh19 | 2022-06-22 | Build site. |
| html | da2c0fe | chenh19 | 2022-06-21 | Build site. |
| html | 8aa5960 | chenh19 | 2022-06-21 | Build site. |
| Rmd | 3dea9b1 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 6783fa3 | chenh19 | 2022-06-21 | Build site. |
| Rmd | 6699b8f | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | d9be701 | chenh19 | 2022-06-21 | Build site. |
| Rmd | f93179d | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 82e6e50 | chenh19 | 2022-06-21 | Build site. |
| Rmd | f753baa | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | d376ad0 | chenh19 | 2022-06-21 | Build site. |
| Rmd | 4a9db6a | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 325a212 | chenh19 | 2022-06-21 | Build site. |
| Rmd | d54cc77 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | e14c55c | chenh19 | 2022-06-21 | Build site. |
| Rmd | 2ab041a | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | f971bbd | chenh19 | 2022-06-21 | Build site. |
| html | dca882f | chenh19 | 2022-06-21 | Build site. |
| html | 3a80eaf | chenh19 | 2022-06-21 | Build site. |
| Rmd | b6fb1d2 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 33829a5 | chenh19 | 2022-06-21 | Build site. |
| Rmd | a949aec | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 5b446cf | chenh19 | 2022-06-21 | Build site. |
| Rmd | c4ad45d | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 28a06ee | chenh19 | 2022-06-21 | Build site. |
| Rmd | 53f2292 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | d5b4ff0 | chenh19 | 2022-06-21 | Build site. |
| Rmd | e982ac7 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 16400a7 | chenh19 | 2022-06-21 | Build site. |
| Rmd | d6aa5b2 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | a324166 | chenh19 | 2022-06-21 | Build site. |
| Rmd | ab8cbc3 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 27a4d51 | chenh19 | 2022-06-21 | Build site. |
| Rmd | 624e791 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | f765024 | chenh19 | 2022-06-21 | Build site. |
| html | bc55dbb | chenh19 | 2022-06-21 | Build site. |
| Rmd | 8b66c3b | chenh19 | 2022-06-21 | update |
| html | b3b7ed6 | chenh19 | 2022-06-21 | Build site. |
| Rmd | 405116a | chenh19 | 2022-06-21 | update |
| html | 405d57e | chenh19 | 2022-06-21 | Build site. |
| Rmd | bbbaab0 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | c10a1a8 | chenh19 | 2022-06-21 | Build site. |
| Rmd | a8f7999 | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 2443e38 | chenh19 | 2022-06-21 | Build site. |
| Rmd | 8e0c8ad | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 5d192d2 | chenh19 | 2022-06-21 | Build site. |
| Rmd | 356550c | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | 21e9501 | chenh19 | 2022-06-21 | Build site. |
| Rmd | 4a8f7ee | chenh19 | 2022-06-21 | wflow_publish("./analysis/*.Rmd") |
| html | b002776 | chenh19 | 2022-06-20 | Build site. |
| Rmd | 6d78198 | chenh19 | 2022-06-20 | wflow_publish("./analysis/*.Rmd") |
| Rmd | 0e1817a | chenh19 | 2022-06-19 | update |
| html | 6211c60 | chenh19 | 2022-06-15 | Build site. |
| Rmd | 46e8cc3 | chenh19 | 2022-06-15 | wflow_publish("./analysis/*.Rmd") |
| html | 0da18e2 | chenh19 | 2022-06-15 | Build site. |
| Rmd | d999122 | chenh19 | 2022-06-15 | wflow_publish("./analysis/*.Rmd") |
| html | 0aff555 | chenh19 | 2022-06-15 | Build site. |
| Rmd | eda2d56 | chenh19 | 2022-06-15 | wflow_publish("./analysis/*.Rmd") |
| html | a4e1e73 | chenh19 | 2022-06-15 | Build site. |
| Rmd | 7229c17 | chenh19 | 2022-06-15 | wflow_publish("./analysis/*.Rmd") |
| html | f0e98f9 | chenh19 | 2022-06-14 | Build site. |
| Rmd | e0aa022 | chenh19 | 2022-06-14 | wflow_publish("./analysis/*.Rmd") |
| html | eafa16b | chenh19 | 2022-06-14 | Build site. |
| Rmd | 69b29f1 | chenh19 | 2022-06-14 | wflow_publish("./analysis/*.Rmd") |
| html | dfd60ce | chenh19 | 2022-06-14 | Build site. |
| Rmd | 49f1922 | chenh19 | 2022-06-14 | wflow_publish("./analysis/*.Rmd") |
| html | fd7271e | chenh19 | 2022-06-14 | Build site. |
| html | 1b4d12e | chenh19 | 2022-06-14 | Build site. |
| html | a6c402d | chenh19 | 2022-06-14 | Build site. |
| html | cedad99 | chenh19 | 2022-06-14 | Build site. |
| Rmd | 6b3f021 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | e3b9788 | chenh19 | 2022-06-14 | Build site. |
| html | aed5eed | chenh19 | 2022-06-14 | Build site. |
| Rmd | c552123 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 45bb6ed | chenh19 | 2022-06-14 | Build site. |
| Rmd | 81fdf42 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | c23765a | chenh19 | 2022-06-14 | Build site. |
| html | b5fb71c | chenh19 | 2022-06-14 | Build site. |
| Rmd | 10f6641 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 152325b | chenh19 | 2022-06-14 | Build site. |
| Rmd | 1739dd9 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 543ef4c | chenh19 | 2022-06-14 | Build site. |
| html | 9b6cb27 | chenh19 | 2022-06-14 | Build site. |
| Rmd | d8908c0 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 8674c8a | chenh19 | 2022-06-14 | Build site. |
| Rmd | 2972ce6 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | ada4068 | chenh19 | 2022-06-14 | Build site. |
| Rmd | 7c5402e | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 0d08121 | chenh19 | 2022-06-14 | Build site. |
| Rmd | 6226526 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | dd2046d | chenh19 | 2022-06-14 | Build site. |
| Rmd | 71f5d04 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 2c4cab1 | chenh19 | 2022-06-14 | Build site. |
| Rmd | 6e73c04 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | d46eaab | chenh19 | 2022-06-14 | Build site. |
| Rmd | be56d9d | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 02a0c26 | chenh19 | 2022-06-14 | Build site. |
| Rmd | e2c15c0 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | abad46e | chenh19 | 2022-06-14 | Build site. |
| Rmd | 68948e3 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 741027b | chenh19 | 2022-06-14 | Build site. |
| Rmd | 065d7e9 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | bb15812 | chenh19 | 2022-06-14 | Build site. |
| Rmd | b6e0993 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 93a27ae | chenh19 | 2022-06-14 | Build site. |
| Rmd | b4a7331 | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 27121a9 | chenh19 | 2022-06-14 | Build site. |
| Rmd | fce1ffd | chenh19 | 2022-06-14 | wflow_publish("analysis/*.Rmd") |
| html | 44517c1 | chenh19 | 2022-06-13 | Build site. |
| Rmd | cc6a40a | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | da08f11 | chenh19 | 2022-06-13 | Build site. |
| Rmd | 05ccc35 | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | 572f6ba | chenh19 | 2022-06-13 | Build site. |
| html | b8870d3 | chenh19 | 2022-06-13 | Build site. |
| html | 719925e | chenh19 | 2022-06-13 | Build site. |
| html | e7541fa | chenh19 | 2022-06-13 | Build site. |
| html | 9d9615d | chenh19 | 2022-06-13 | Build site. |
| Rmd | 04feaa7 | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | bbd8978 | chenh19 | 2022-06-13 | Build site. |
| Rmd | 0ec2bfa | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | e5a5b52 | chenh19 | 2022-06-13 | Build site. |
| Rmd | c43ae1f | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | 4d8bd72 | chenh19 | 2022-06-13 | Build site. |
| html | 3373521 | chenh19 | 2022-06-13 | Build site. |
| html | af21ea8 | chenh19 | 2022-06-13 | Build site. |
| Rmd | 6e56d75 | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | f653f7b | chenh19 | 2022-06-13 | Build site. |
| Rmd | 2723e7f | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | d69c892 | chenh19 | 2022-06-13 | Build site. |
| html | 34d877d | chenh19 | 2022-06-13 | Build site. |
| html | e72400b | chenh19 | 2022-06-13 | Build site. |
| html | c411223 | chenh19 | 2022-06-13 | Build site. |
| html | 1daccd2 | chenh19 | 2022-06-13 | Build site. |
| Rmd | 63f46d2 | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | 26adb45 | chenh19 | 2022-06-13 | Build site. |
| html | a6022a8 | chenh19 | 2022-06-13 | Build site. |
| Rmd | 1215832 | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | 9abc4b8 | chenh19 | 2022-06-13 | Build site. |
| Rmd | 7efcfe0 | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | f18d385 | chenh19 | 2022-06-13 | Build site. |
| Rmd | a7c1ce0 | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | e991f56 | chenh19 | 2022-06-13 | Build site. |
| html | 3c9b1d9 | chenh19 | 2022-06-13 | Build site. |
| Rmd | ae1553a | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | 34e0d02 | chenh19 | 2022-06-13 | Build site. |
| Rmd | e69aa83 | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | 9be31af | chenh19 | 2022-06-13 | Build site. |
| Rmd | ead84c2 | chenh19 | 2022-06-13 | wflow_publish("analysis/*.Rmd") |
| html | 0f41de8 | chenh19 | 2022-06-13 | Build site. |
| html | 31ad035 | chenh19 | 2022-06-13 | Build site. |
| html | bdf3b44 | chenh19 | 2022-06-13 | Build site. |
| html | 8d0890c | chenh19 | 2022-06-13 | Build site. |
| Rmd | 26f455b | chenh19 | 2022-06-13 | update |
| html | 26f455b | chenh19 | 2022-06-13 | update |
1. Understand RNA-seq
a. Read about RNA-seq analysis
Yalamanchili et al. 2017: RNA-seq analysis pipeline
Some key points:
Protocol-1 (differential expression of genes):
- demuxed raw reads (FastQC)
- trimming reads (awk)
- aligning reads (TopHat2)
- counting reads (HTSeq; may filter out genes with low counts before next step)
- detect DE using counted reads (DEseq2)
- more QC (PCA/correlation heatmap)
Protocol-2 (differential usage of isoforms):
- Protocol-1
- counting isoforms (Kallisto, also check cell ranger)
- detect DU using counted isoforms (Sleuth)
- more QC (aslo PCA/correlation heatmap)
Protocol-3 (crypic splicing):
- Protocol-1
- detect differential junstions (CrypSplice)
b. Read more about RNA-seq analysis
Luecken
et al. 2019: RNA-seq analysis pipeline
Supplementary
code
c. Read the Morris paper
Some key points:
Some key ideas:
- STING-seq: Systematic Targeting and Inbition of Noncoding GWAS loci with scRNA-seq
- prioritizes candidate cis-regulatory elements (cCREs, 1kb<distance to TSS<1Mb) using fine-mapped GWAS
- selected 88 variants (in 56 loci) with enhancer activity
- dual CRISPR inhibition: dCas9 as the GPS, MeCP2 and KRAB as the repressors
- confirming dual CRISPRi efficacy: gRNAs target TSS of MRPS23, CTSB, FSCN1
- CRIPSRi on the 88 variants: two gRNAs for each variant, both within 200bp of the variant
- ECCITE-seq: captures gRNAs and epitopes
Some data processing steps and results:
- QC: remove cells with low total reads or excessive mitochondrial reads, gRNA assignment UMI>5 (9,343 cells after QC)
- Kallisto: counting read more on the official website
- Seurat: QC and
reference mapping?read more on the official website - SCEPTRE: gRNA_to_gene-expression pairwise test
- non-targeting gRNA-gene pairs: not significant (negative ctrl)
- TSS-targeting gRNA-gene pairs: expression significantly decreased (positive ctrl)
- 37 of the 88 variants were significant
- Trans-regulatory elements:
I'll come back later
Note:
- Cellular Indexing of Transcriptomes and Epitopes by Sequencing (CITE-seq)
- Expanded CRISPR-compatible CITE-seq (ECCITE-Seq)
- cDNA, HTO (Hashtag oligos), GDO (gRNAs)
- ECCITE-seq:

2. Prelim QC for raw STING-seq data
a. Download all data
Code: download.sh
b. Perform FastQC on all fastq files
Code: fastqc.sh
SRR14141135:
SRR14141136:
SRR14141137:
SRR14141138:
SRR14141139:
SRR14141140:
SRR14141141:
SRR14141142:
SRR14141143:
SRR14141144:
SRR14141145:
SRR14141146:
A brief summary:
- length:
26bpor57bp(trimmed?) - depth:
30-35x - overall quality: good (within
~40 bp)
d. Kallisto | bustools pipeline
Code: pip3-kb.sh
Code: anaconda_kallisto.sh
3. Analyze QC’ed STING-seq data
a. Install packages
Code: seurat.sh
b. Data overview
Code: overview.R
Note: about sparse
matrix
The [Expression] matrix has:
- 35,606 rows/genes/targets
- 686,612 columns/barcodes/cells
- 24,447,506,872 values in total
- 82,507,471 values that are non-zero
- 50,421,358 values that are 1
- 32,086,113 values that are bigger than 1
- 3,370,699 values that are bigger than 10
- 259,734 values that are bigger than 100
- 2,515 values that are bigger than 1,000
- 0 values that are bigger than 10,000
- 0 values that are bigger than 100,000
The [gRNA] matrix has:
- 210 rows/genes/targets
- 137,347 columns/barcodes/cells
- 28,842,870 values in total
- 2,506,474 values that are non-zero
- 1,510,919 values that are 1
- 995,555 values that are bigger than 1
- 121,554 values that are bigger than 10
- 41,071 values that are bigger than 100
- 2,232 values that are bigger than 1,000
- 20 values that are bigger than 10,000
- 0 values that are bigger than 100,000
The [Hashtag] matrix has:
- 4 rows/genes/targets
- 410,228 columns/barcodes/cells
- 1,640,912 values in total
- 739,820 values that are non-zero
- 409,830 values that are 1
- 329,990 values that are bigger than 1
- 218,280 values that are bigger than 10
- 8,155 values that are bigger than 100
- 282 values that are bigger than 1,000
- 46 values that are bigger than 10,000
- 0 values that are bigger than 100,000
c. Calculate means and non-zeros
Code: Expression_barcode_stats.R
Code: Expression_target_stats.R
Code: gRNA_barcode_stats.R
Code: gRNA_target_stats.R
Code: Hashtag_barcode_stats.R
Code: Hashtag_target_stats.R
d. Expression (cDNA) dataset
i) Expression barcodes
Code: Expression_barcode_dist_plot.R
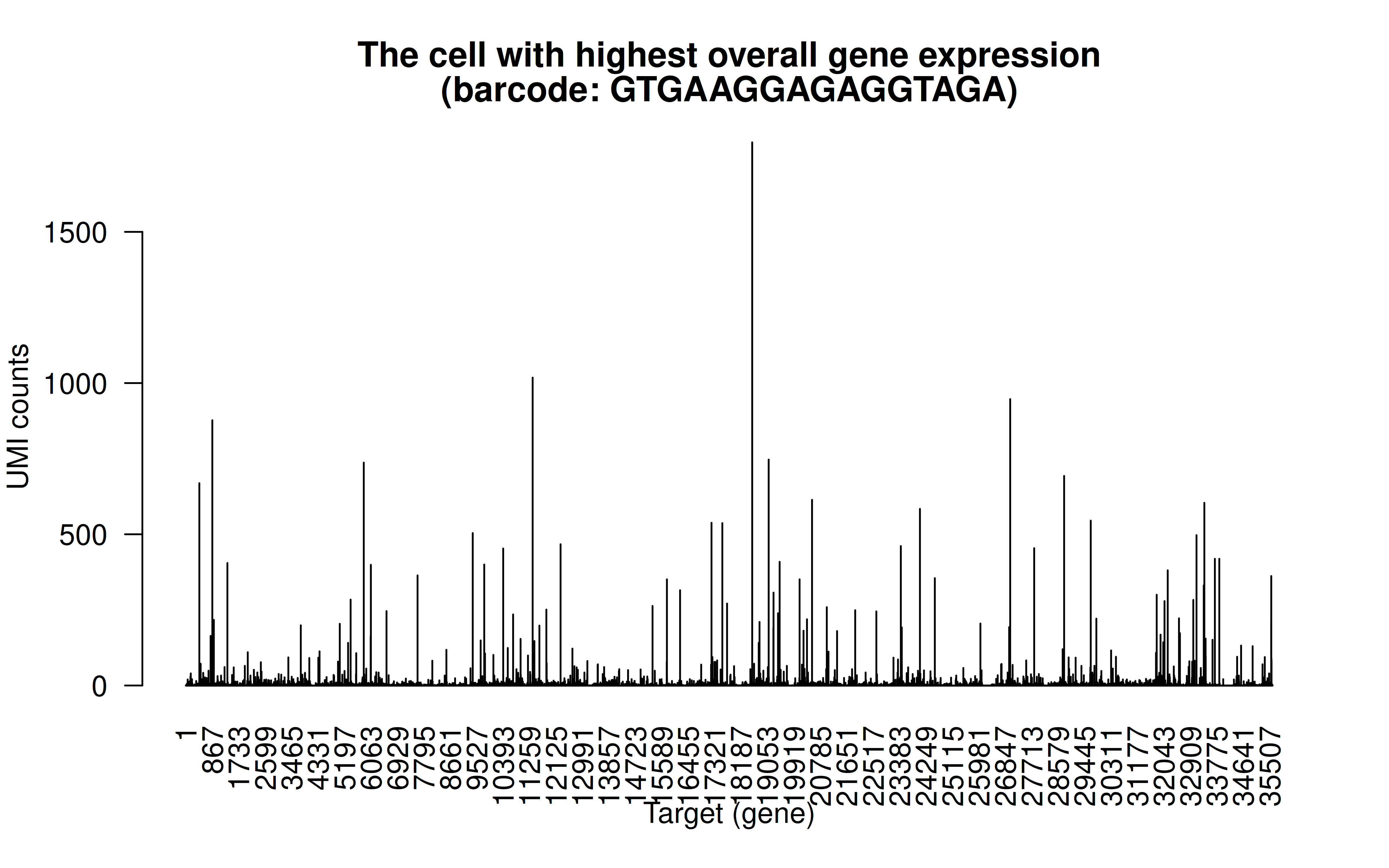
Comment: The cell with highest overall detected gene expression
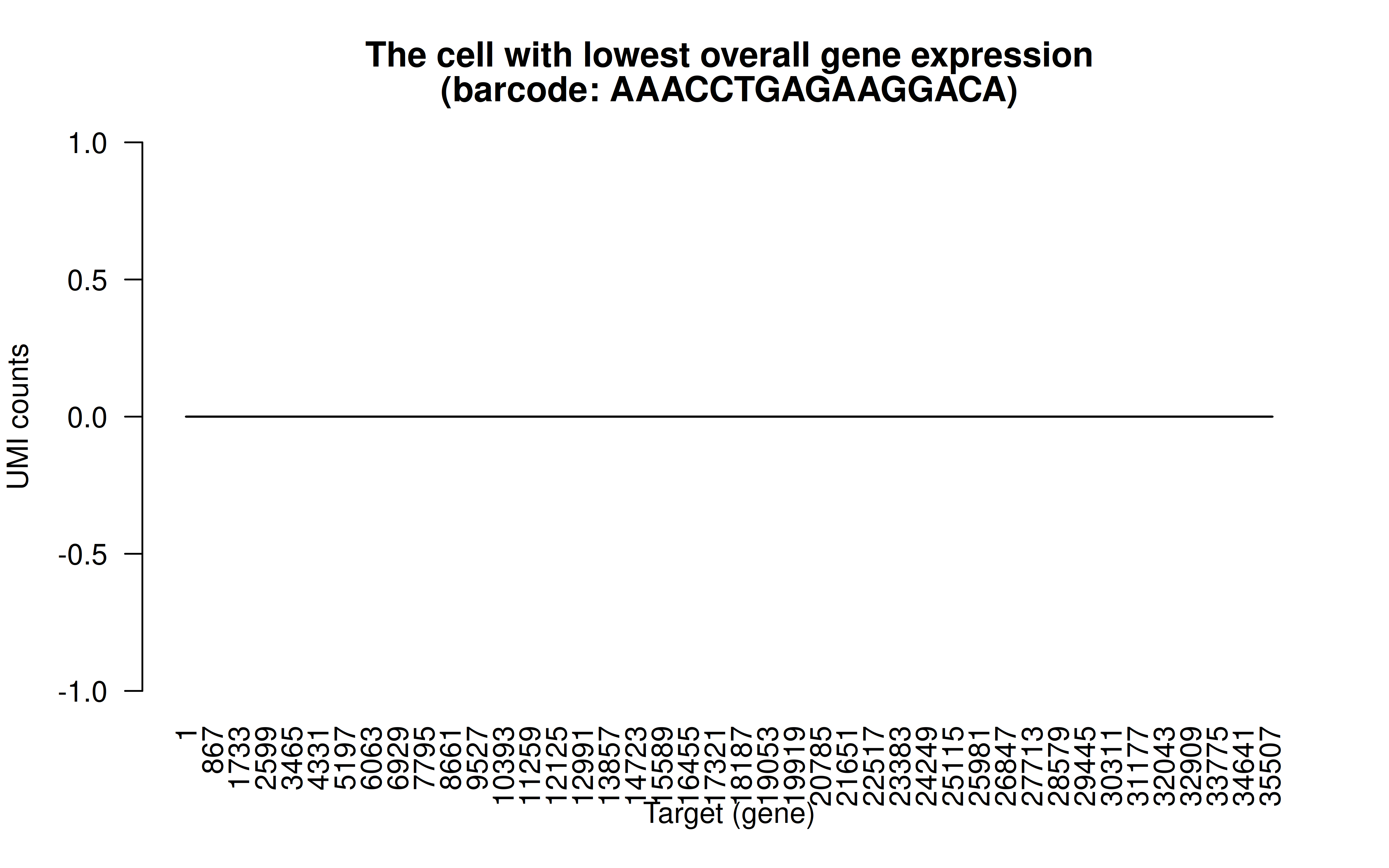
Comment: The cell with lowest overall detected gene expression
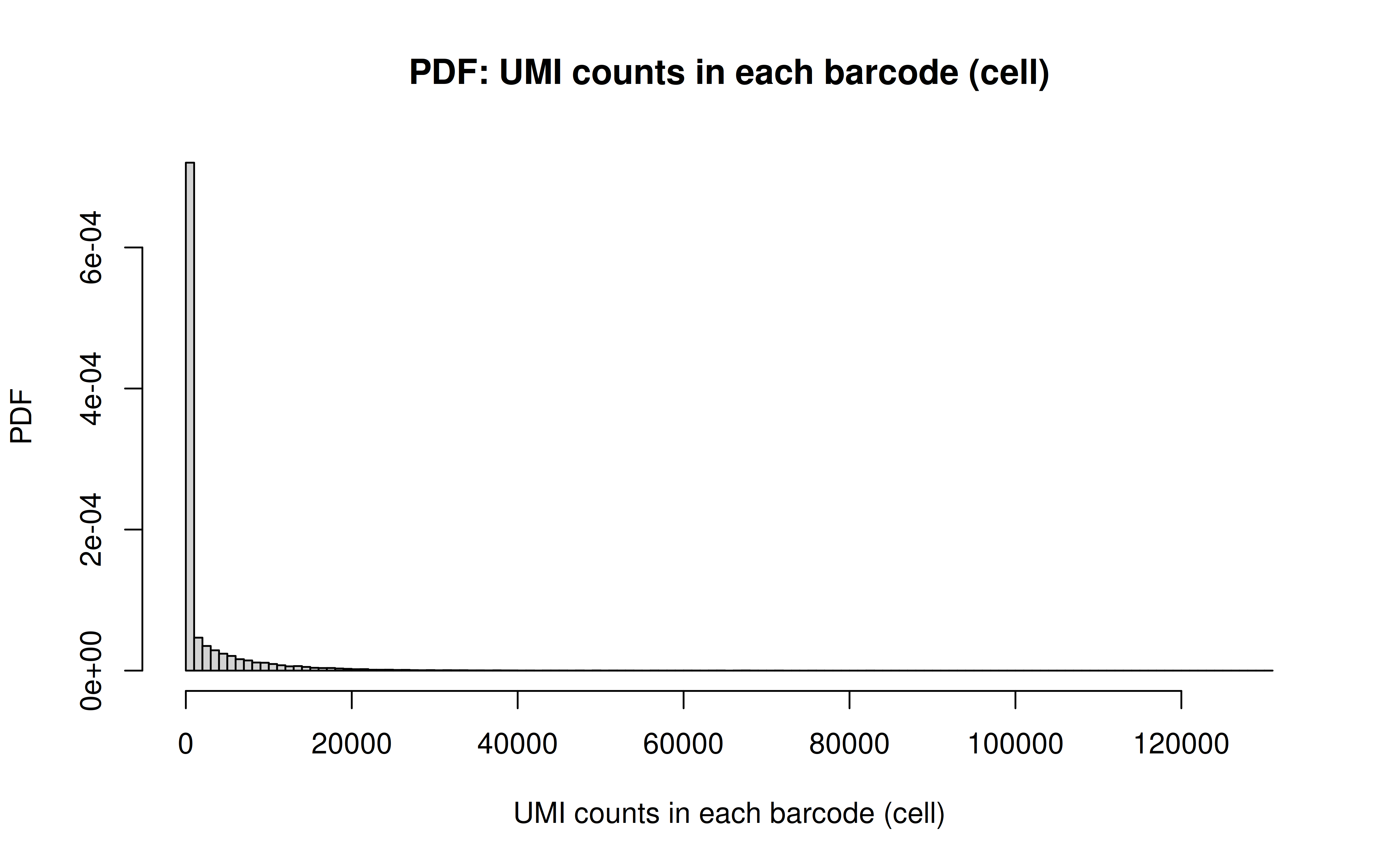
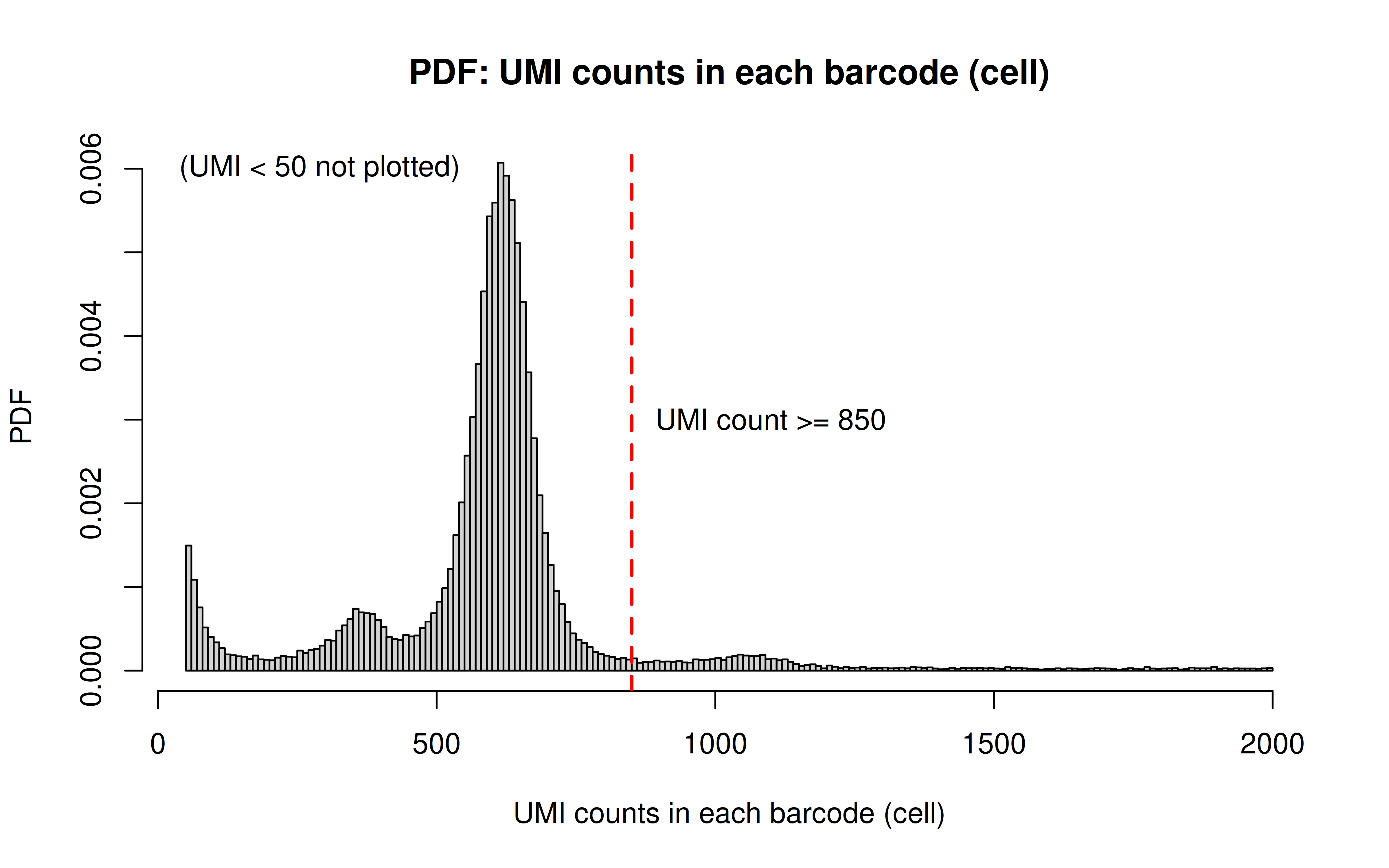
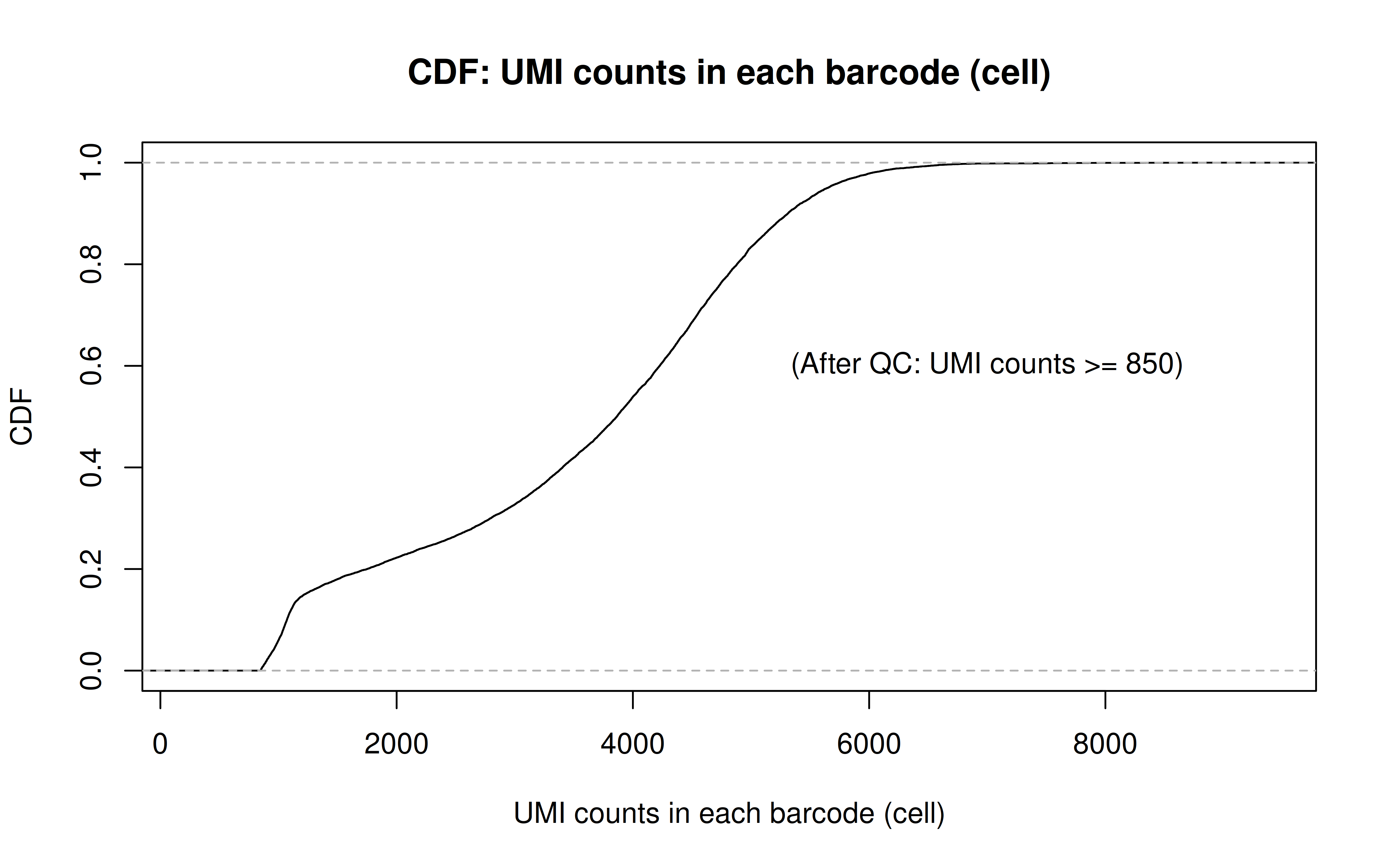
Comment: As Xuanyao said, this kind bar plot is too dense and can’t really see the overall distribution, the CDF plot below is more clear
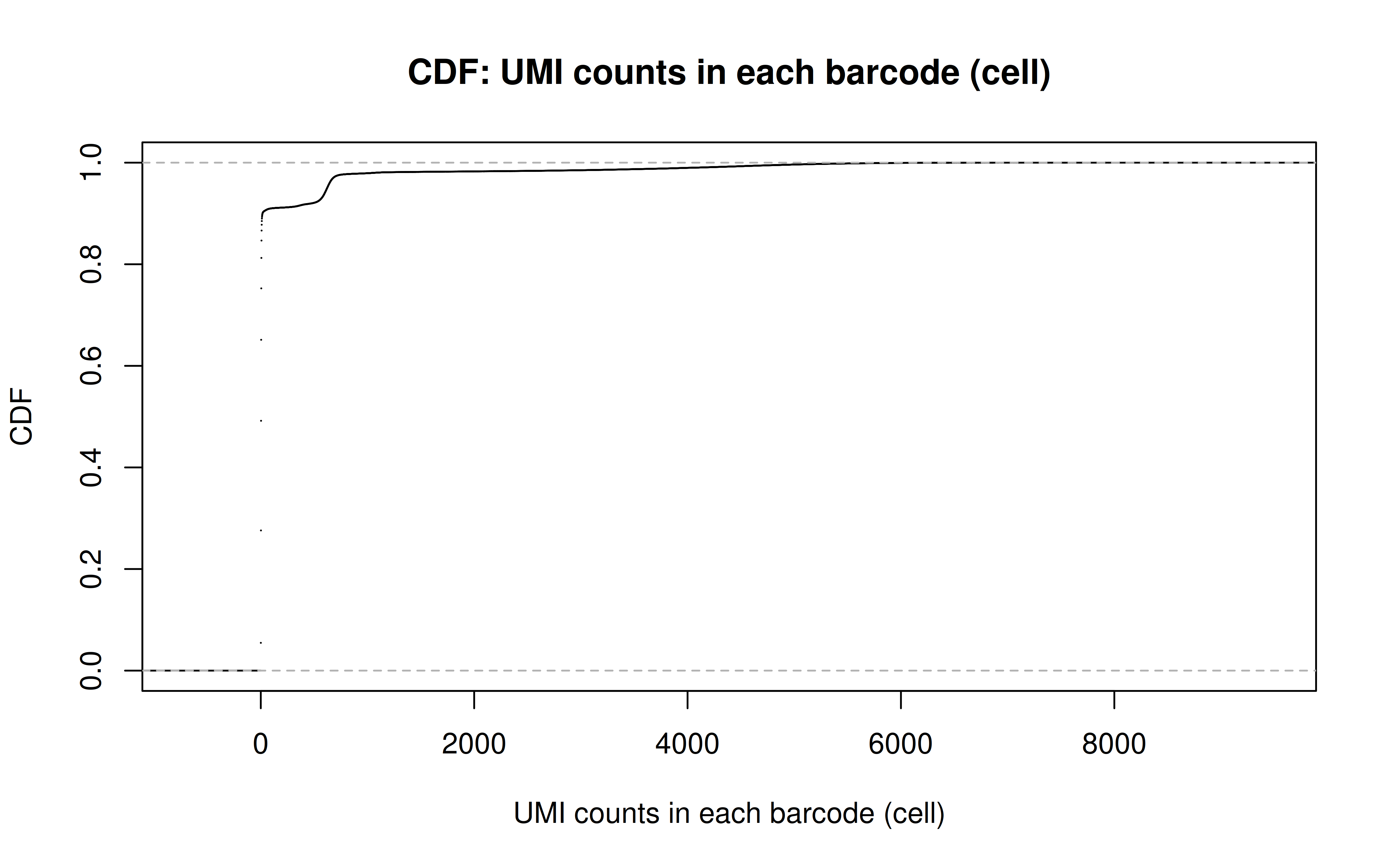
Comment: From this CDF figure I kind of know why there were only ~9000 cells used after Qc’ing with UMI>=850 filter. Less than 10% cells have UMI>=850. But still, why exactly 850 is still a question for me to explore
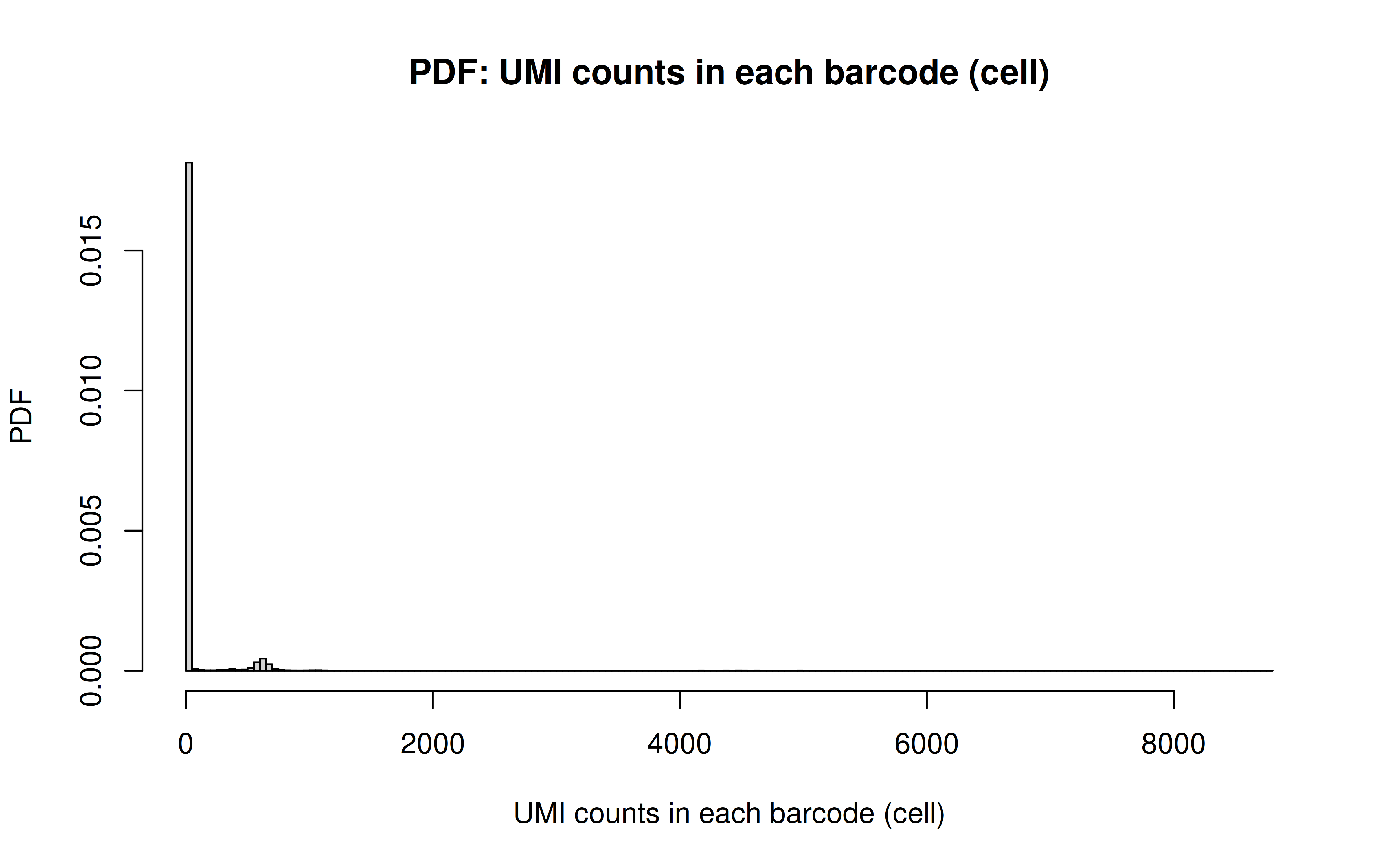
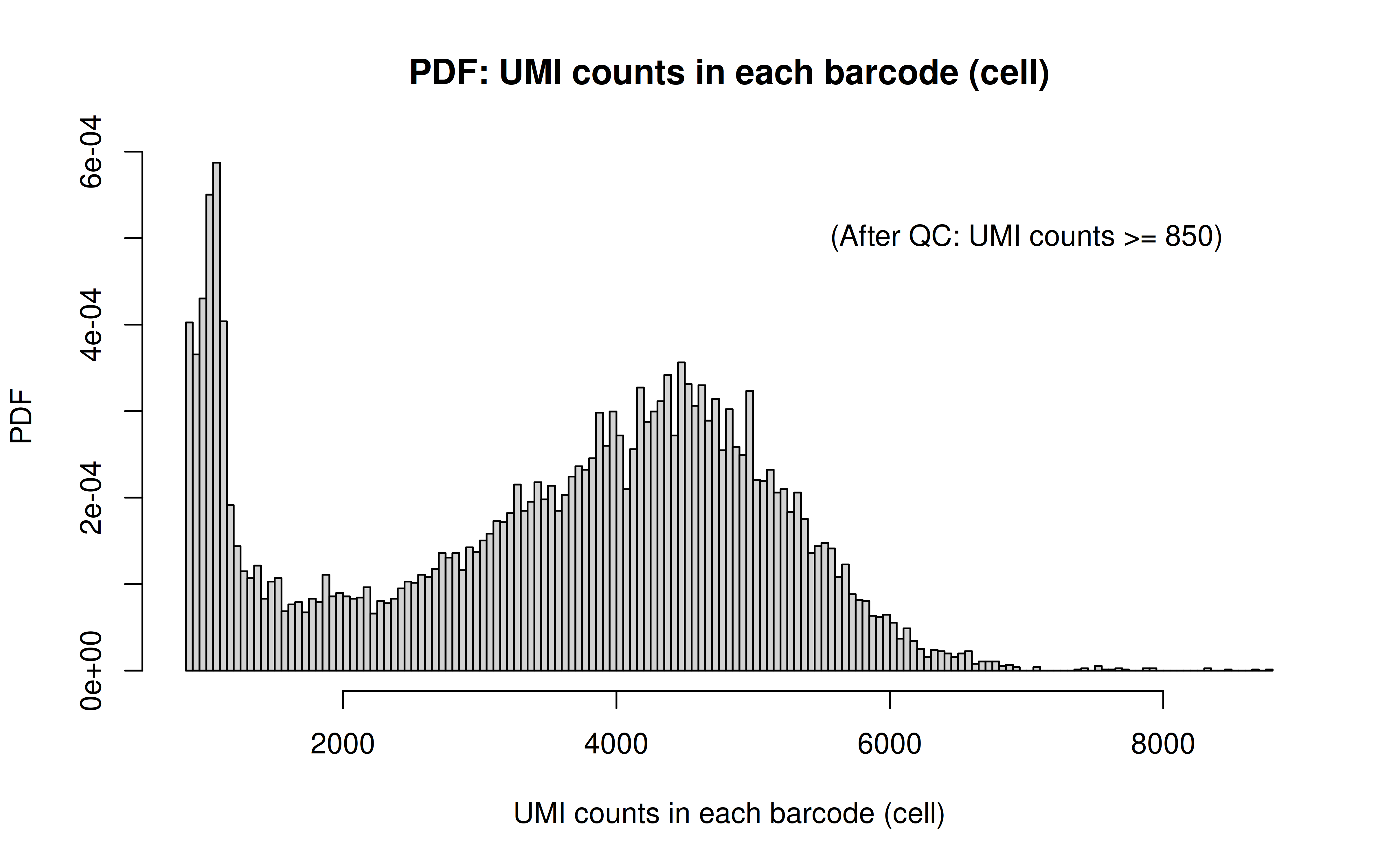
Comment: Many zeros (consistent with the observation that the matrix was very sparse); UMI>850 is invisible in this plot. As Xuanyao said, I should exclude the outliers or add y-axis break
ii) Expression targets
Code: Expression_target_dist_plot.R
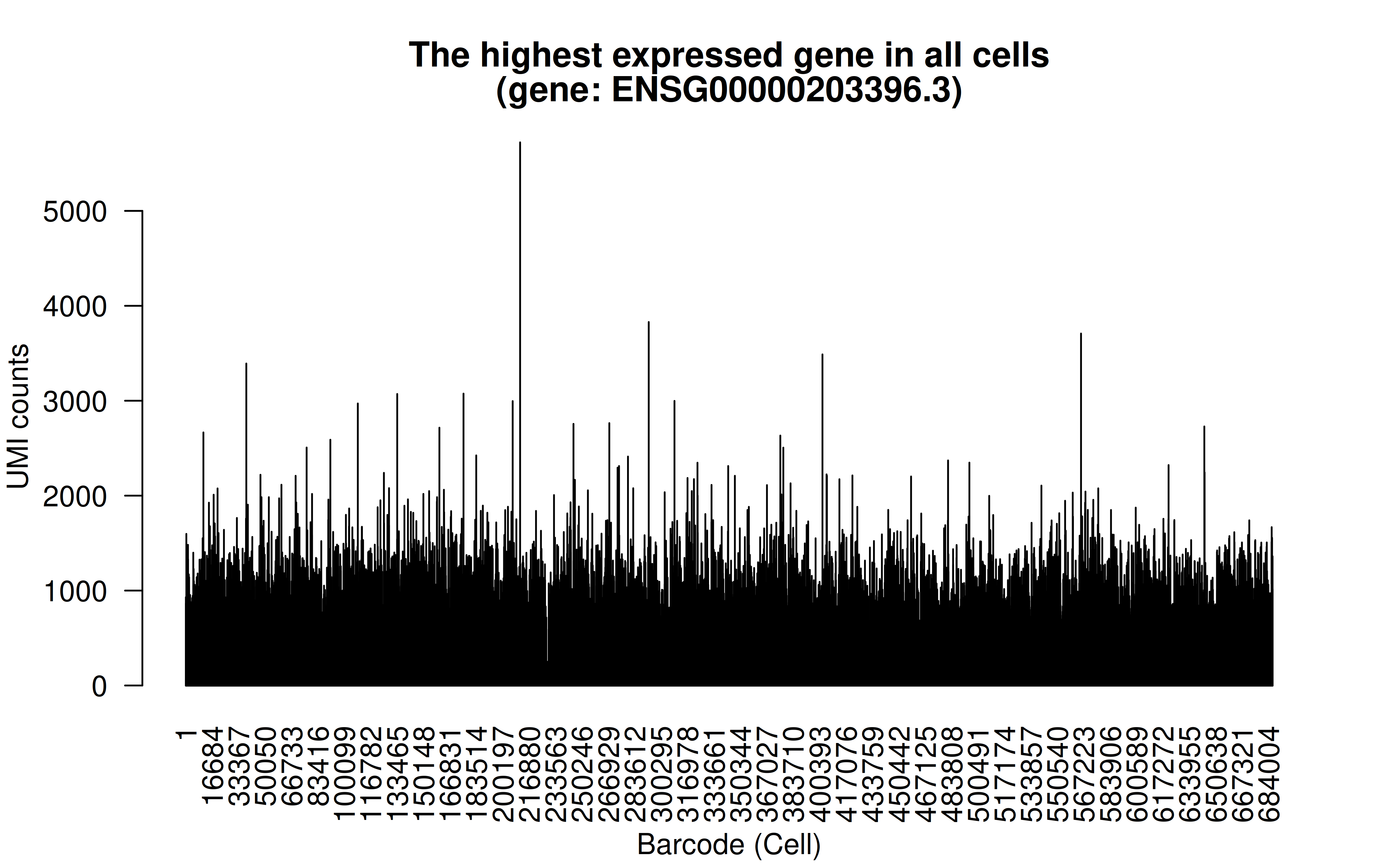
Comment: The highest (mean) expressed gene is WDR45-like (WDR45L) pseudogene (high UMI counts in all cells)
Comment: The lowest (mean) expressed gene is RP4-669L17.1 pseudogene (zero UMI counts in all cells)
Comment: Non-zero UMI counts for all genes (~35k, including mito genes; 686,612 cells intotal)

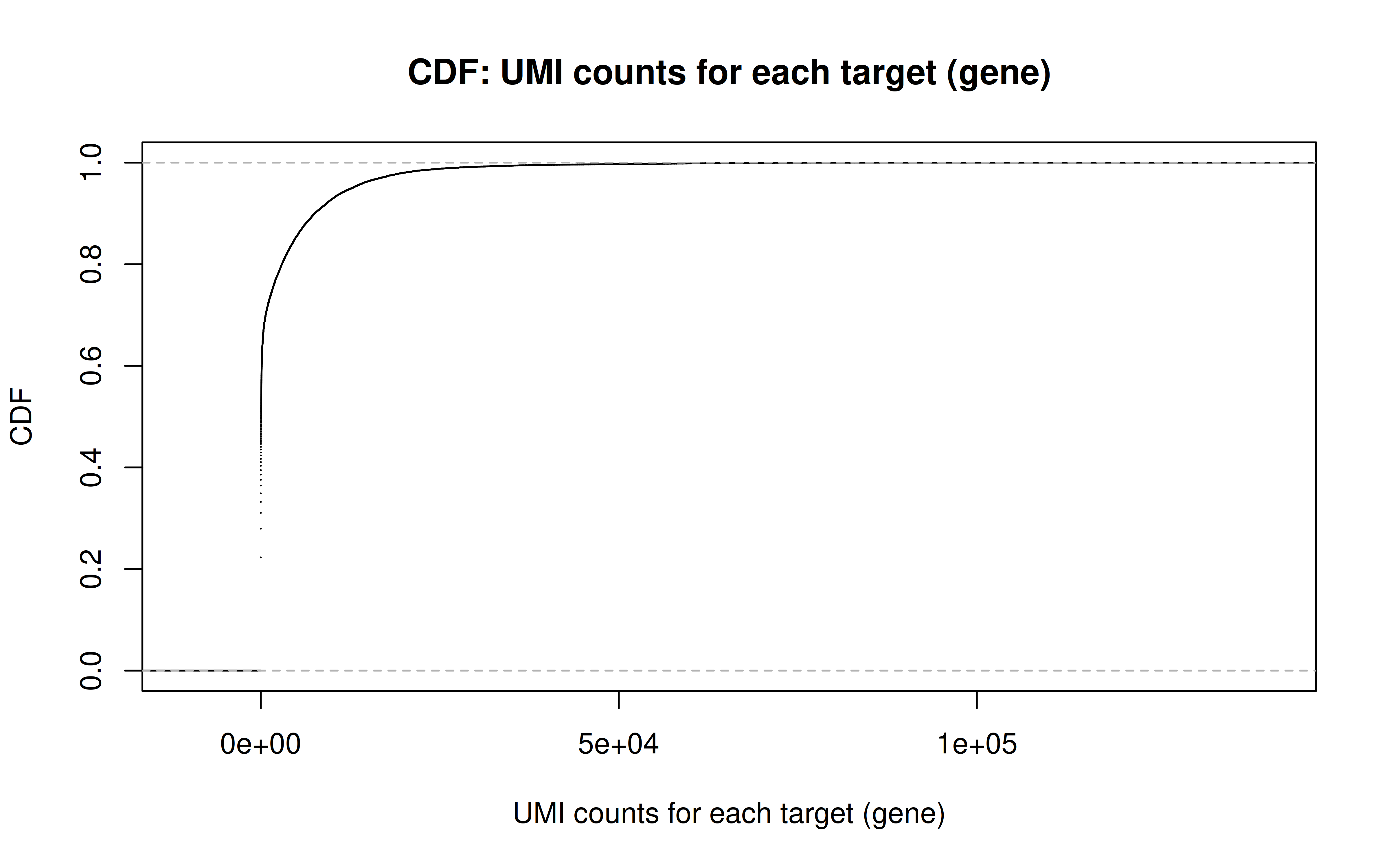
Comment: CDF plot: ~80% genes have < ~5000 UMI counts in all cells (not all genes captured in each cell, but I guess still a lot)
Comment: PDF plot: same conclusion as above
e. gRNA dataset
i) gRNA barcodes
Code: gRNA_barcode_dist_plot.R
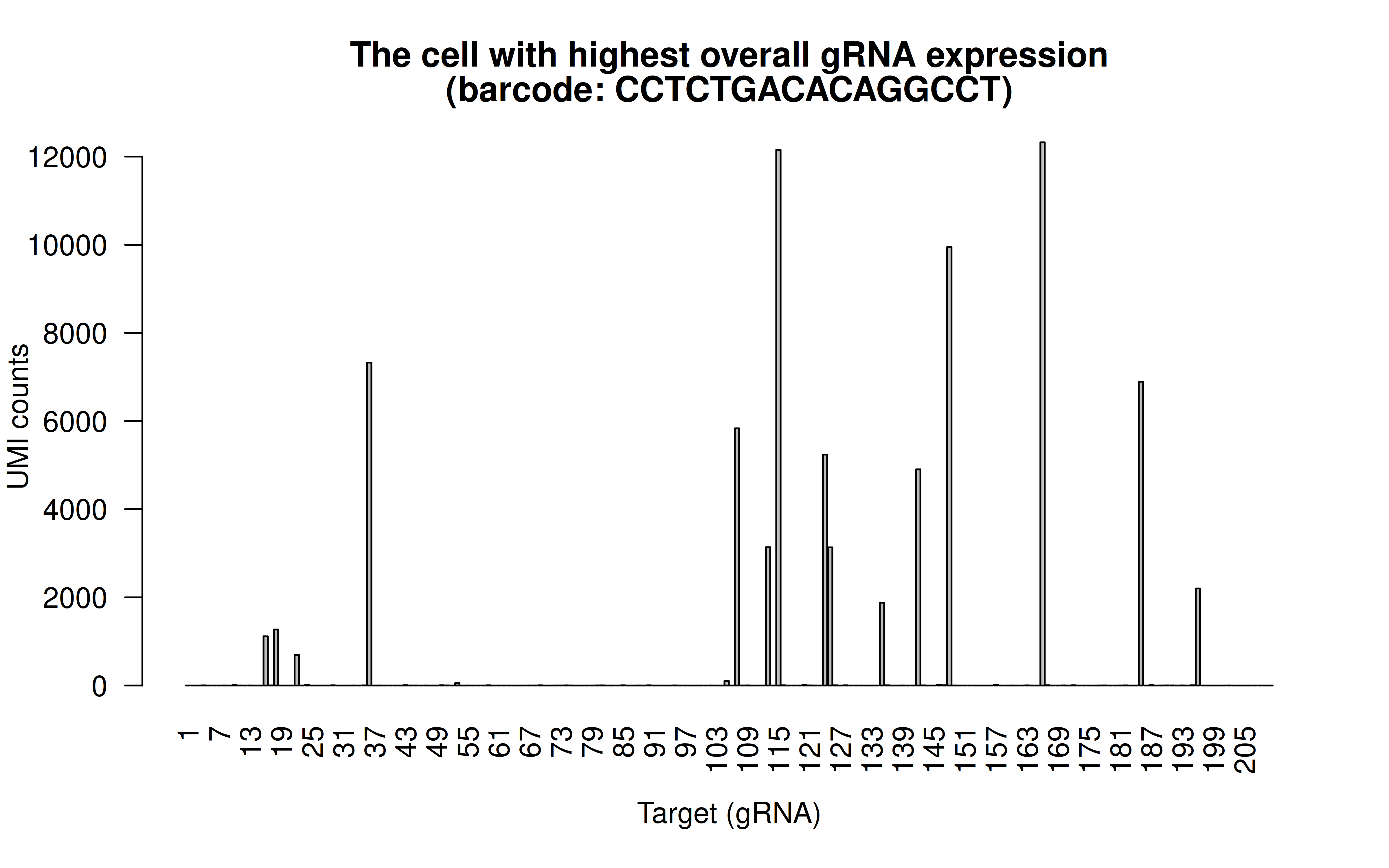
Comment: The cell with highest overall (mean) gRNAs, and it has 15 highly expressed gRNAs
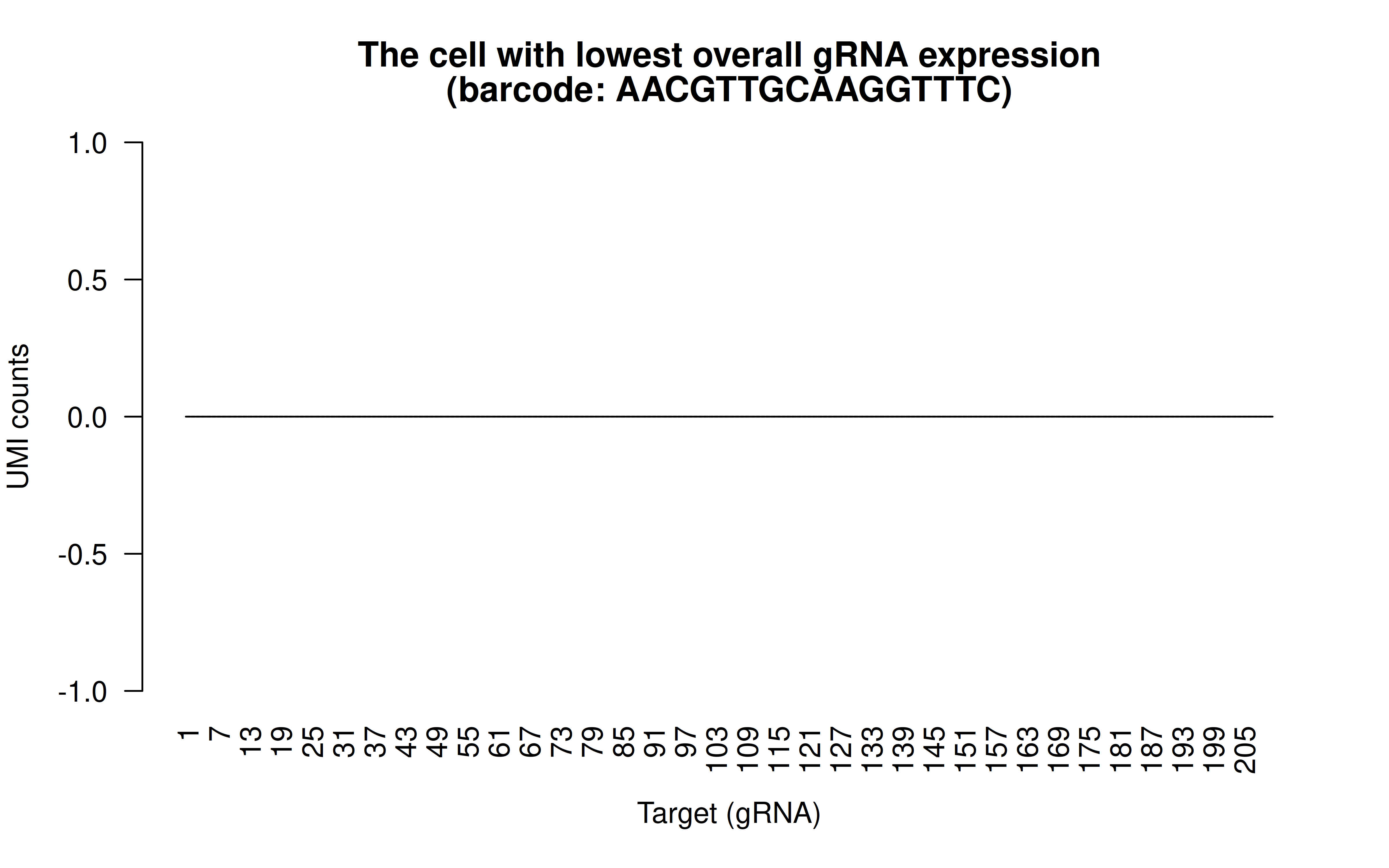
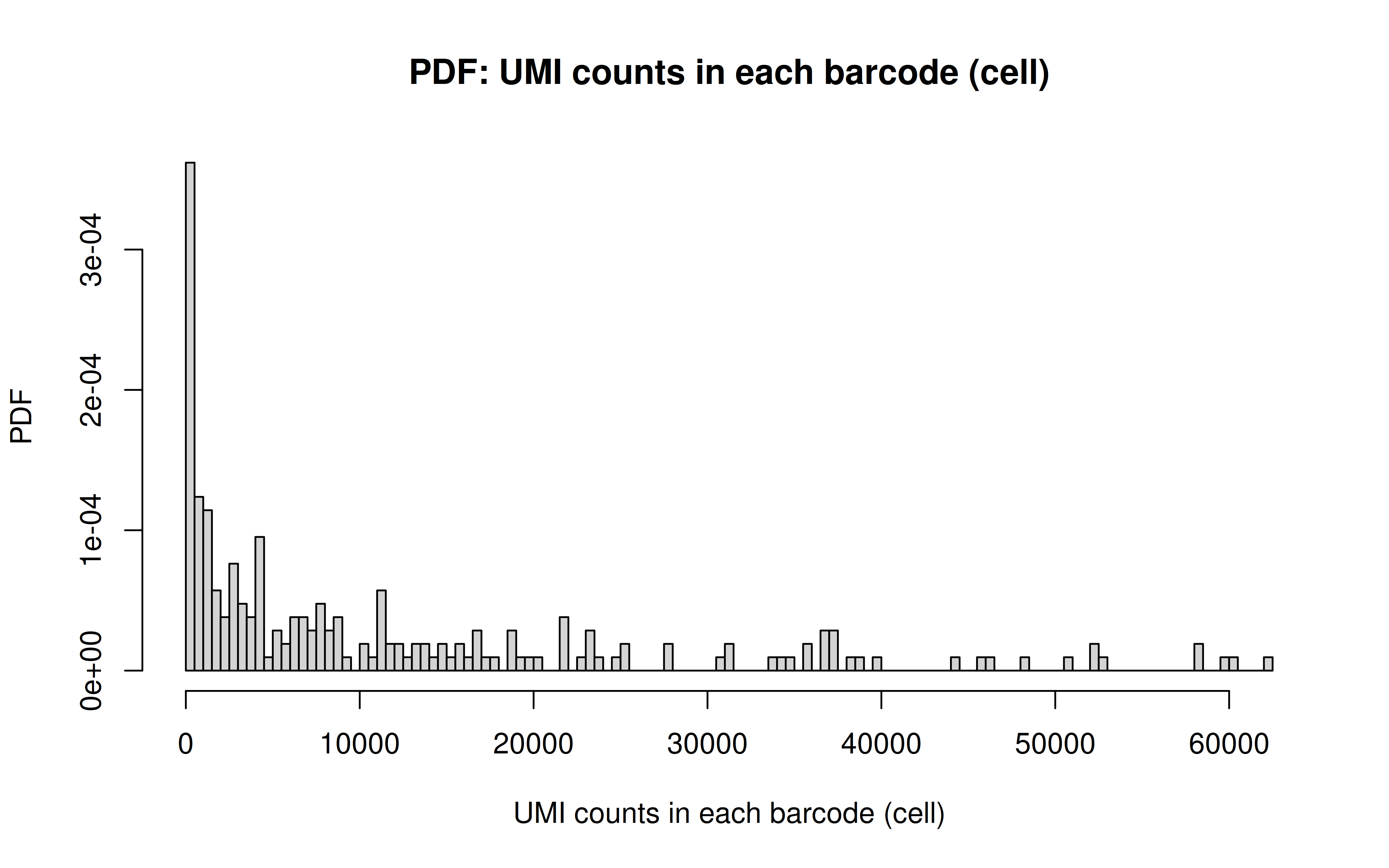
Comment: The cell with lowest overall (mean) gRNAs (transfection/transduction failed in this cell)
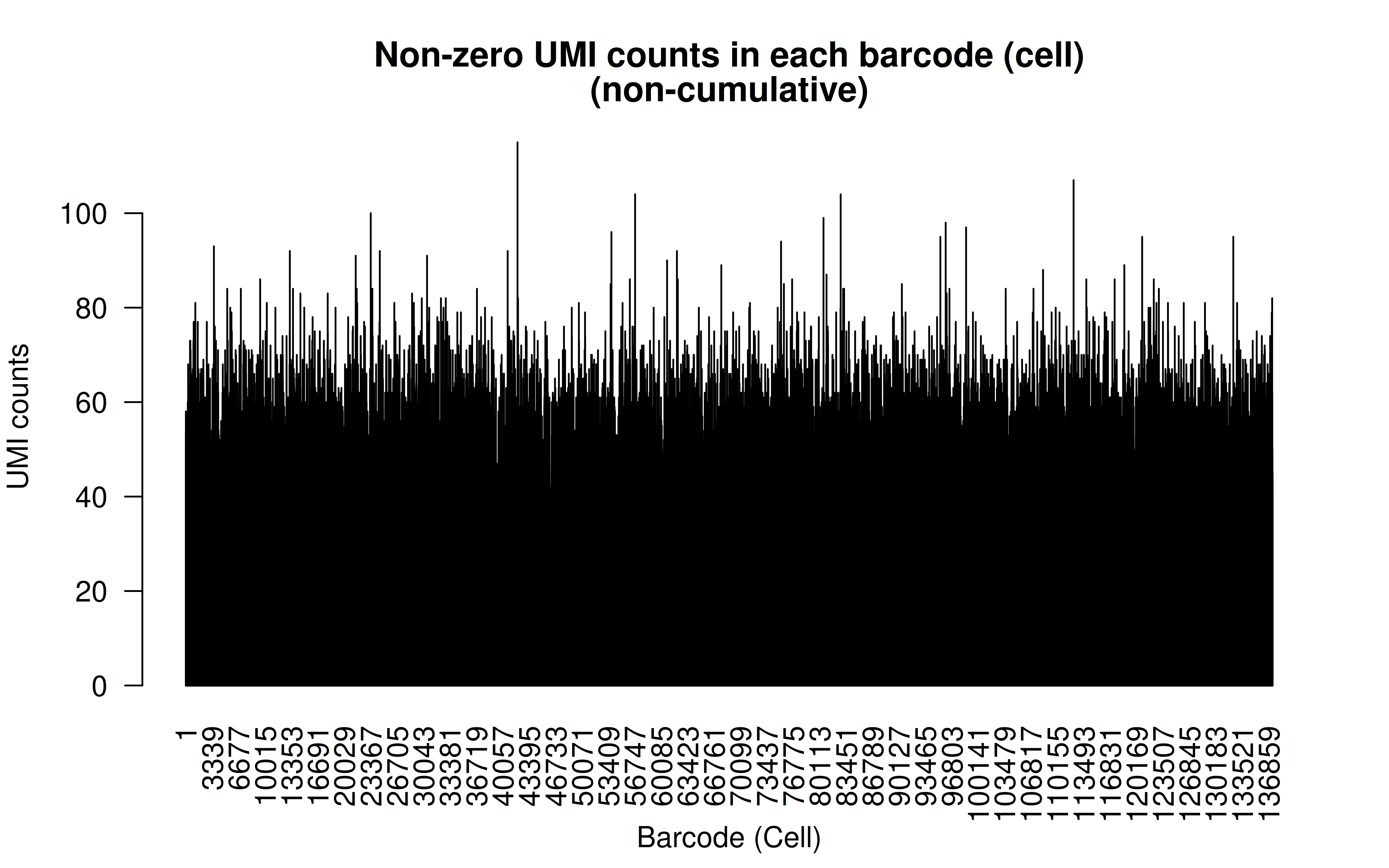
Comment: Non-zero UMI counts in all cells (I’d say the transfection/transduction relatively even across all cells)
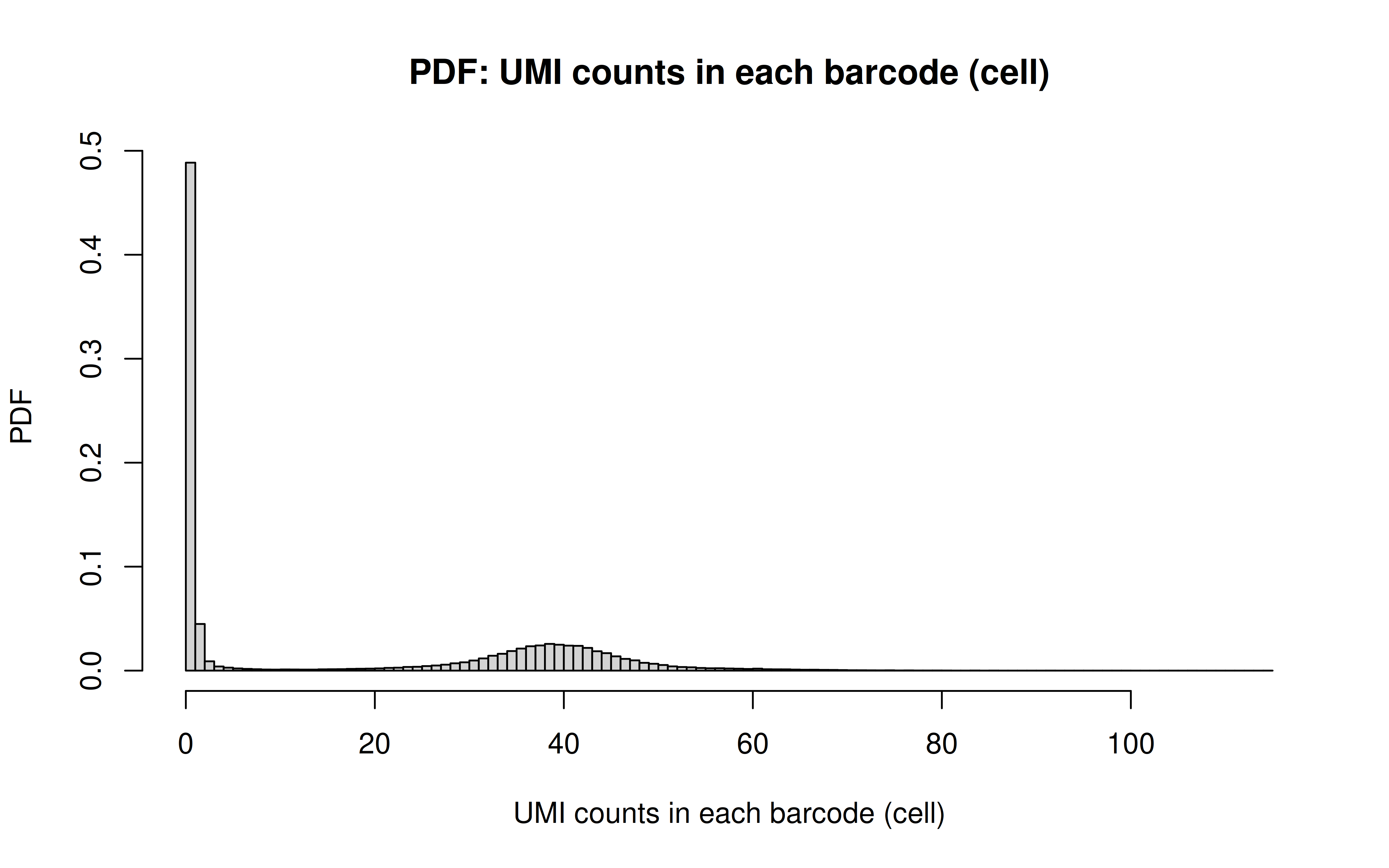
Comment: CDF plot: ~80% cells have < ~40 UMI counts for each gRNA (note: the authors mentioned MOI ~ 10)
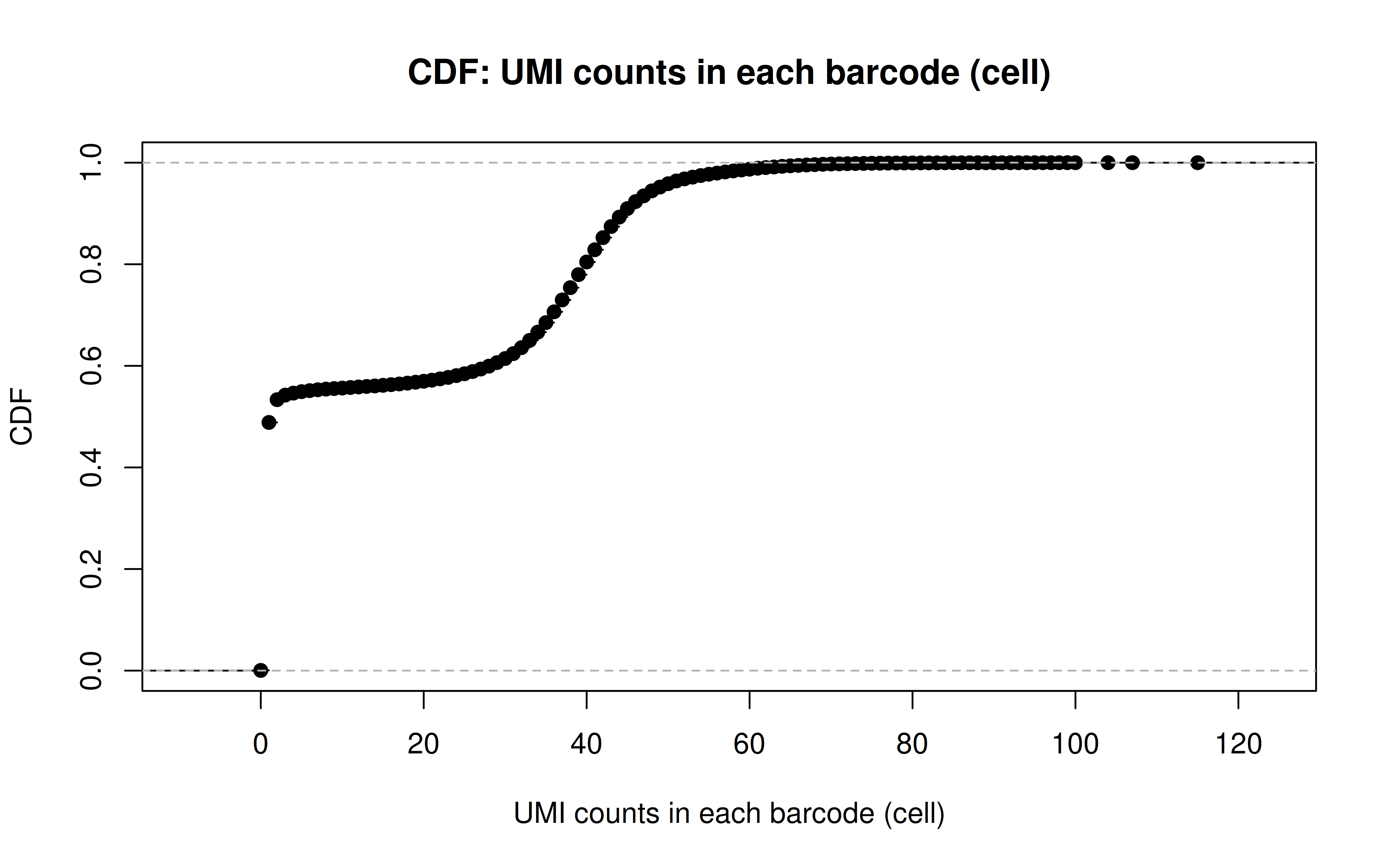
Comment: PDF plot: same conclusion as above
ii) gRNA targets
Code: gRNA_target_dist_plot.R
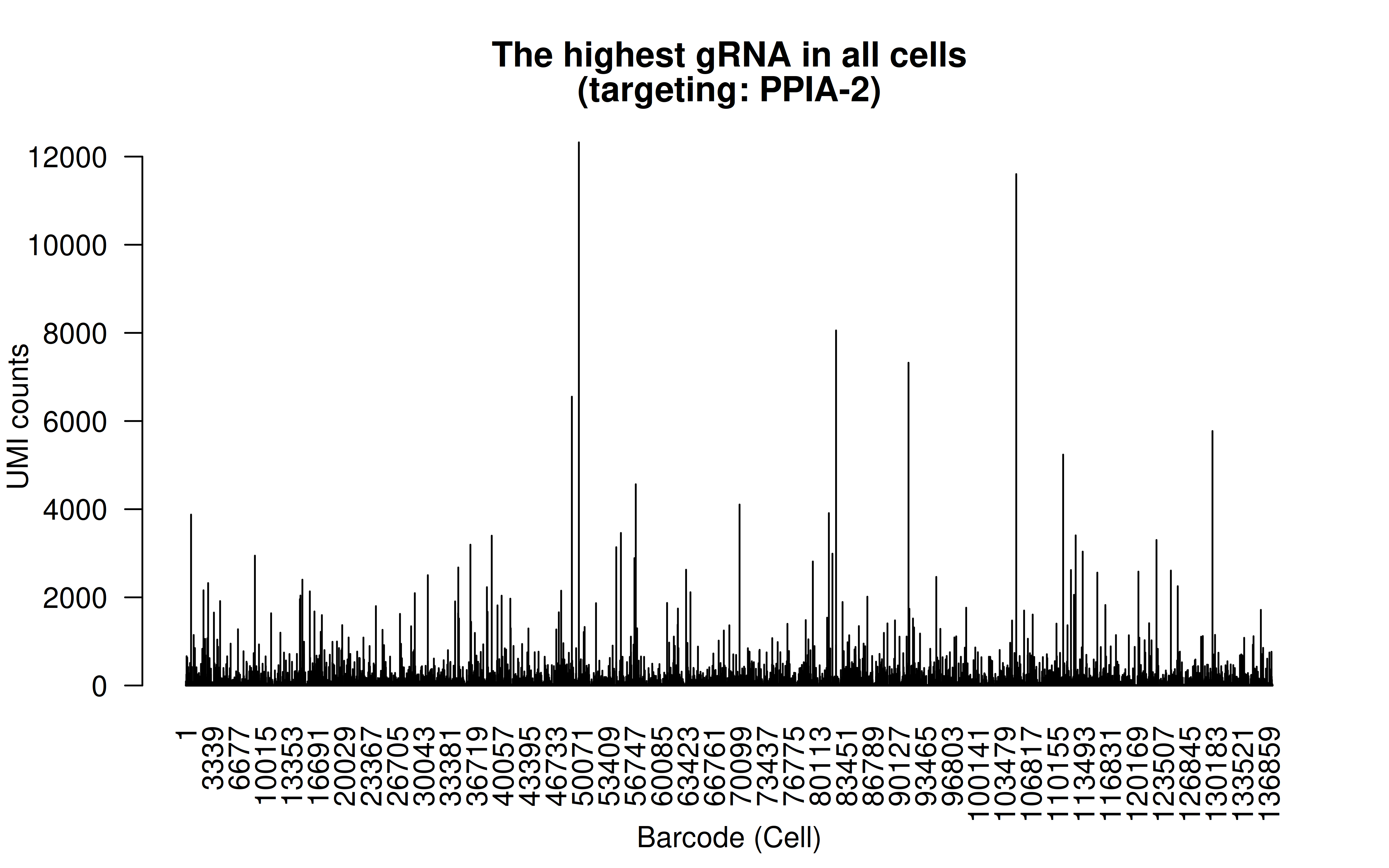
Comment: The highest (mean) gRNA in all cells (gRNA targeting PPIA-2, which is a control)
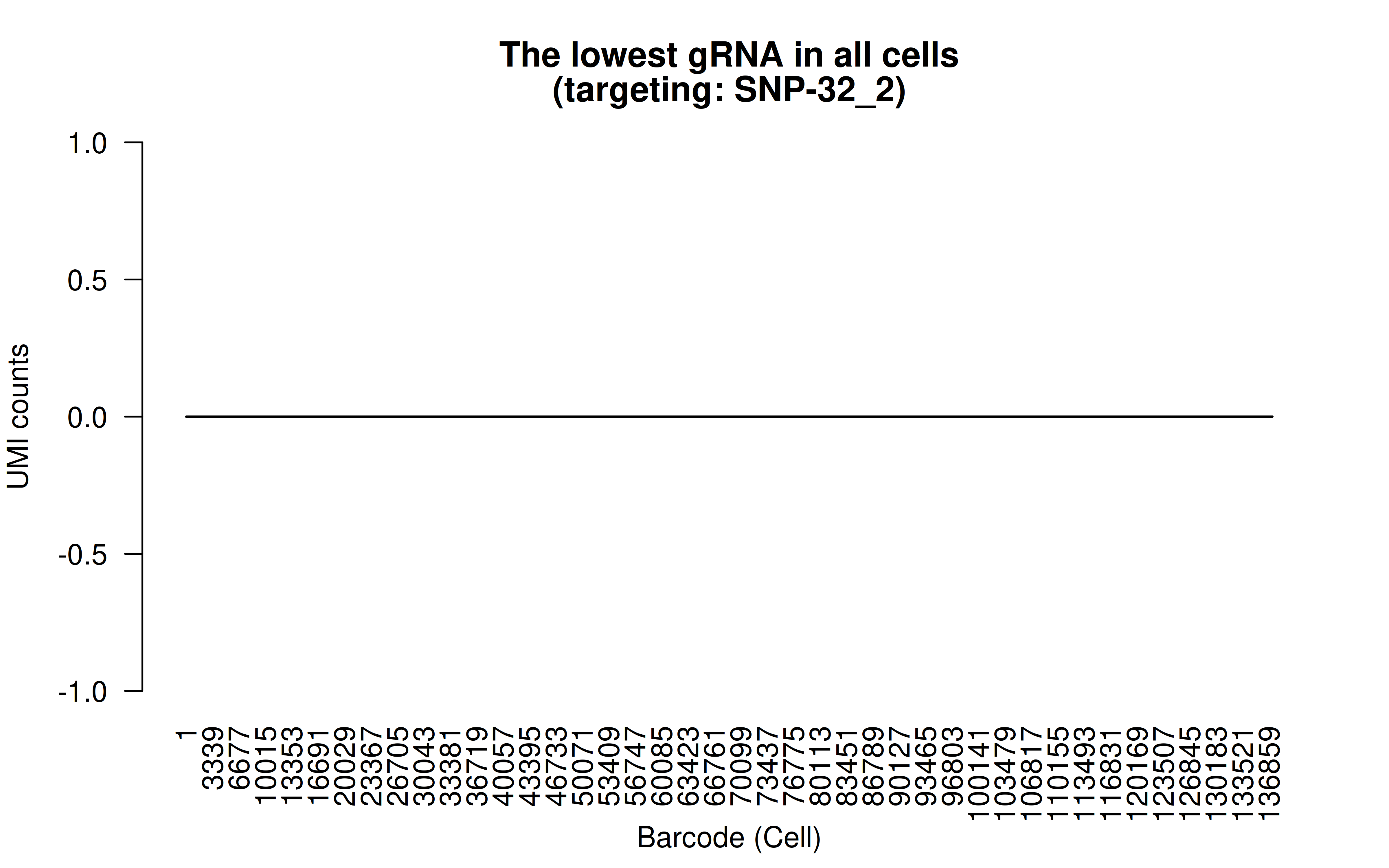
Comment: The lowest (mean) gRNA in all cells (likely it’s a low score gRNA site but the authors didn’t have better choices)
Comment: Non-zero UMI counst for all gRNAs (137,347 cells in total; I’d say the transfection/transduction efficiency varies among gRNAs. The authors designed all the gRNAs within 200bp of the targeted variants,there must be limitations in terms of gRNA options)
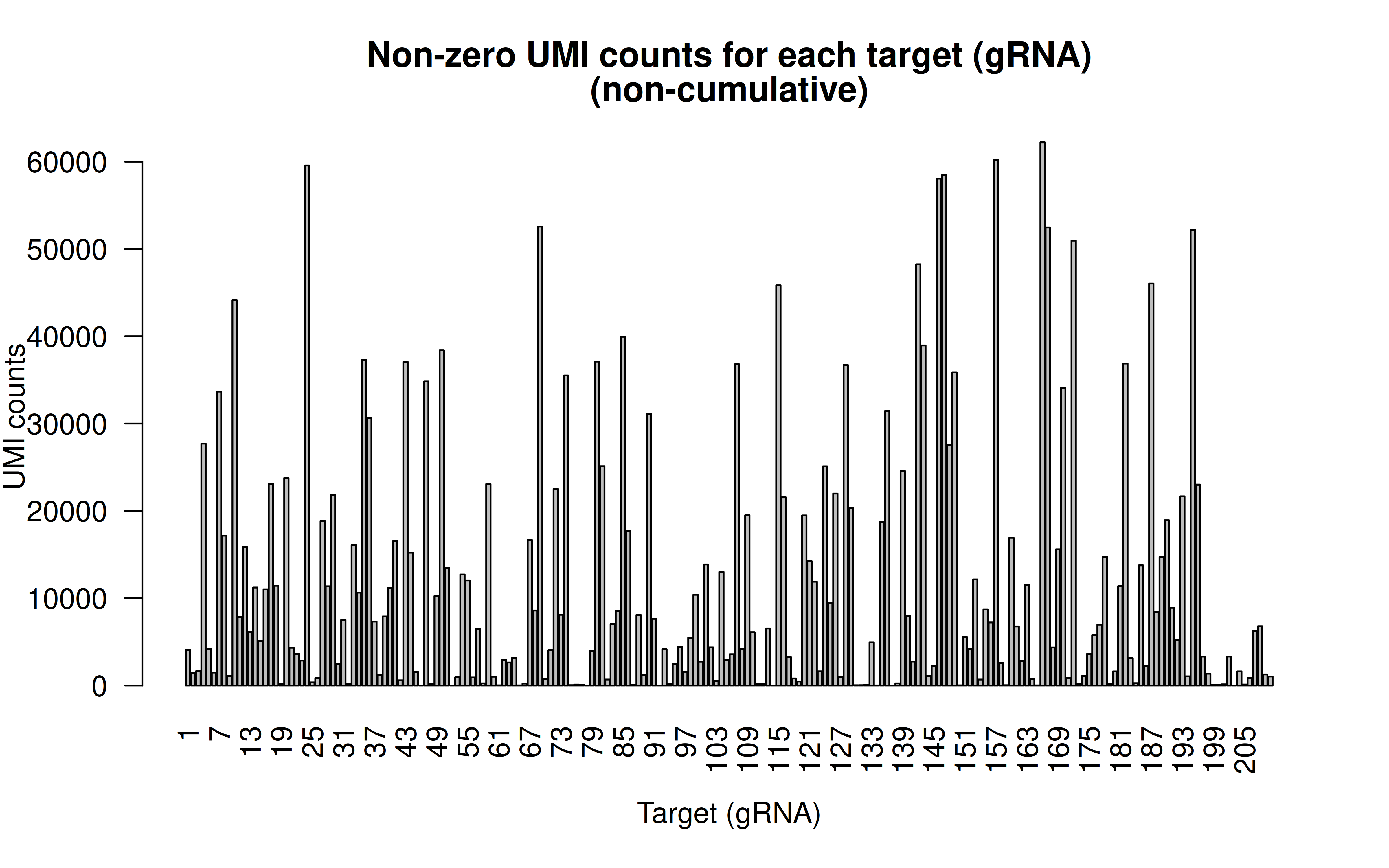
Comment: CDF plot: ~80% gRNAs have < ~20,000 UMI counts in all cells (137,347 cells in total, ~15% transfection/transduction success rate, acceptable)
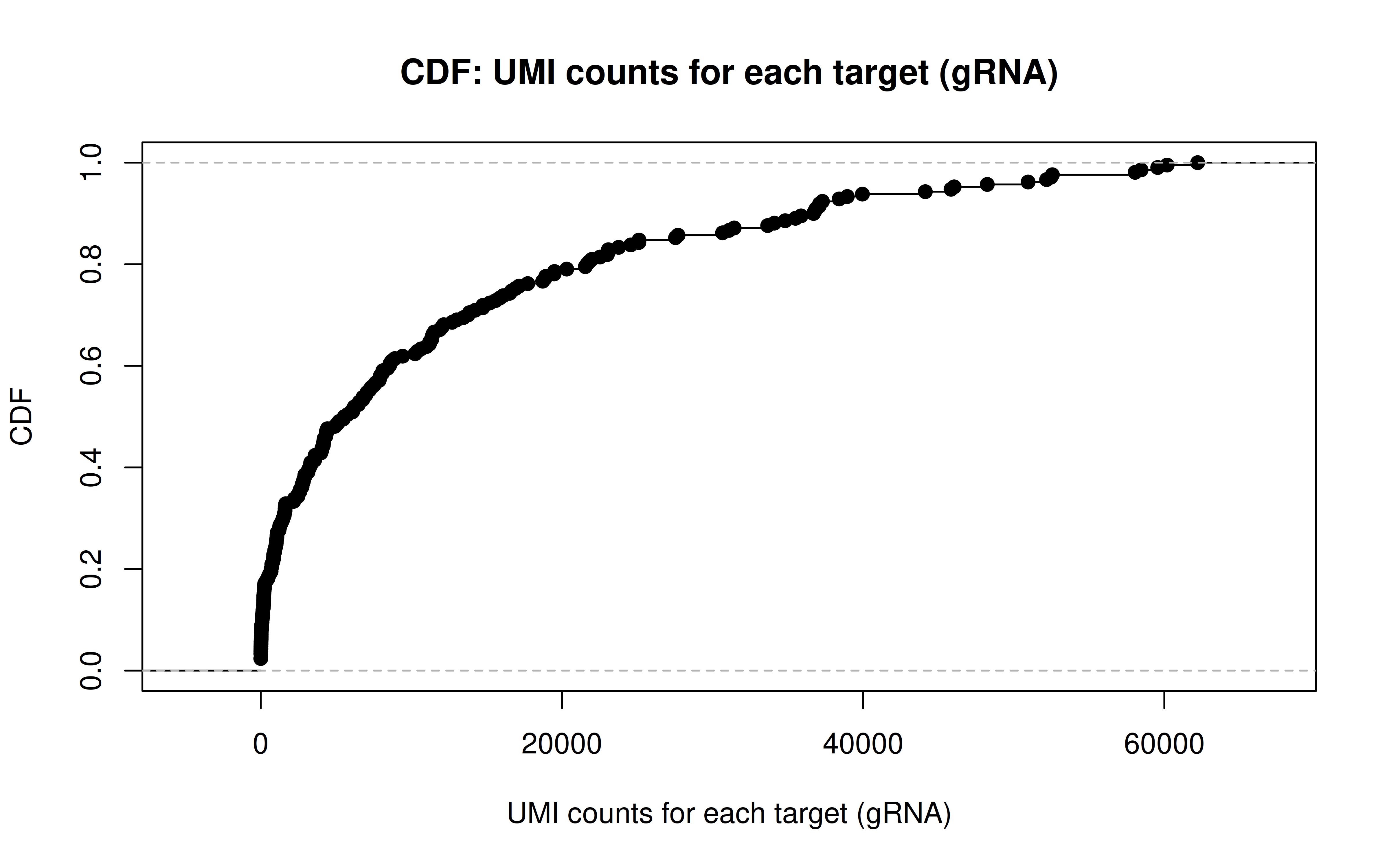
Comment: PDF plot: same conclusion as above
f. Hashtag dataset
i) Hashtag barcodes
Code: Hashtag_barcode_dist_plot.R
Comment: The cell with highest (mean) Hashtags (note: the authors used only 4 Hashtags, I might check which antibodies they are when performing association)

Comment: The cell with lowest (mean) Hashtags (not tagged by any of the antibodies)

Comment: This figure is not an error. All cells have 1/2/3/4 UMI counts, and because many of them have 4, it looks like a block when it’s such dense

Comment: CDF plot: ~80% cells have < ~2 UMI counts for each Hashtag (It make sense to me because the authors are likely trying to label different cell types)

Comment: PDF plot: same conclusion as above

i) Hashtag targets
Code: Hashtag_target_dist_plot.R
Comment: The highest (mean) Hashtag (HTO23) in all cells (I would guess this is the relatively more common cell type, also, there were some non-specific antibody binding)

Comment: The lowest (mean) Hashtag (HTO25) in all cells (I would guess this is the relatively less common cell type, also, it dosen’t seem to overlap with HTO25, which is a good thing)

Comment: Non-zero UMI counts for the 4 Hashtags (I’d say the 4 cell types are relatively even)

Comment: CDF plot: ~80% Hashtags have < ~200,000 UMI counts in all cells (410,228 cells in total, I thinking the antibody binding efficiency is pretty good)

Comment: PDF plot: same conclusion as above

g. QC filtering
- After preliminary filtering, the authors got
14,775 cellswith3,875 median genes per cell.
Previous question:
- why
percent-mito < 20%? - why
UMI > 850? - why
no UMI upper limit?
My understanding:
- Previously, 5% was usually the default threshold. But a recent paper did systematic evaluation and proposed a default cutoff of 10% for human cells. Also, according to Luecken et al. 2019, we can use a relatively loose QC cutoff at the beginning. Since the percent-mito was just in the first QC filtering step and the author further filtered by HTO and GDO, I think 20% makes sense.
- Again, according to Luecken et al. 2019, the dying/dead cells would be a small peak with low UMI counts. By the zoomed-in plot below, we can see 850 is a reasonable cutoff to remove the entire peak.
- Doublets was not filtered out by UMI, but by HTO demuxing, therefore the authors didn’t set an upper limit.
Notes:
- In the original cDNA feature txt file, there are only Ensembl gene IDs. To calculate percent-mito, I tried to convert the IDs to symbols using both this web tool and biomaRT package in R.
- If I directly use the gene symbols from these two methods and the
same filters by Nikita, I get exactly the same
results as Nikita (
14,813 cellsretained). - However, after taking a closer look at the converted gene lists,
there are still many “Mitochondrially Encoded” genes
starting with “
MT” rather than “MT-”, so I wrote a script to convert all these genes. - Then I performed filtering again and got
14,675 cellswith3,917 median genes per cell. - I don’t think we’ll know exactly how the authors filtered the cells unless they release their code.
- After QC filtering, there were
9,391 cellsretained (9,343 cellsby the authors in comparison, - I did cross comparison. There are
508 cellsfrom authors’ list not in my list.
Code: QC_filter.R
Code: UMI_plot.R
Before UMI count filtering:
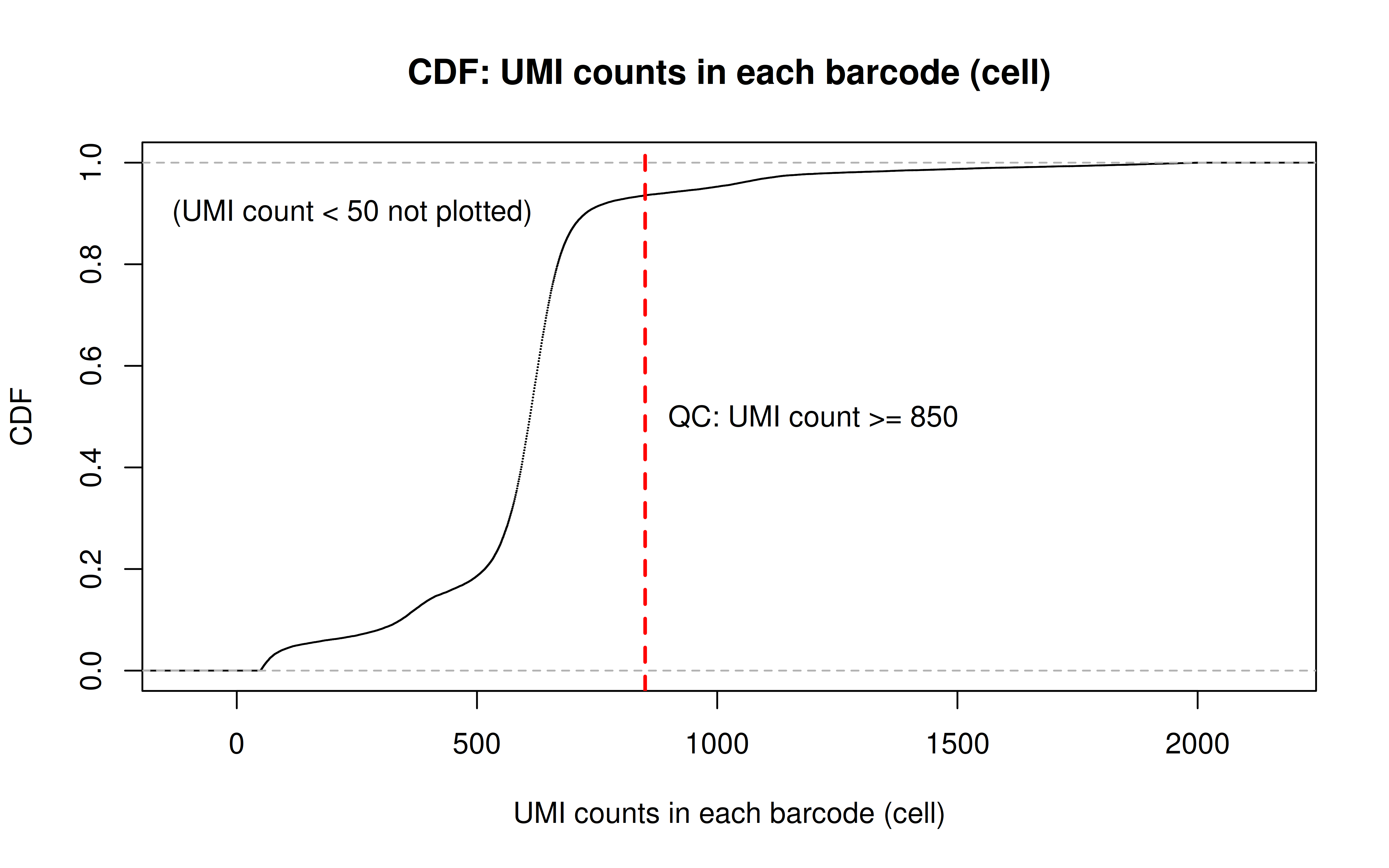
After UMI count filtering:
Before percent-mito filtering (generated by Seurat):
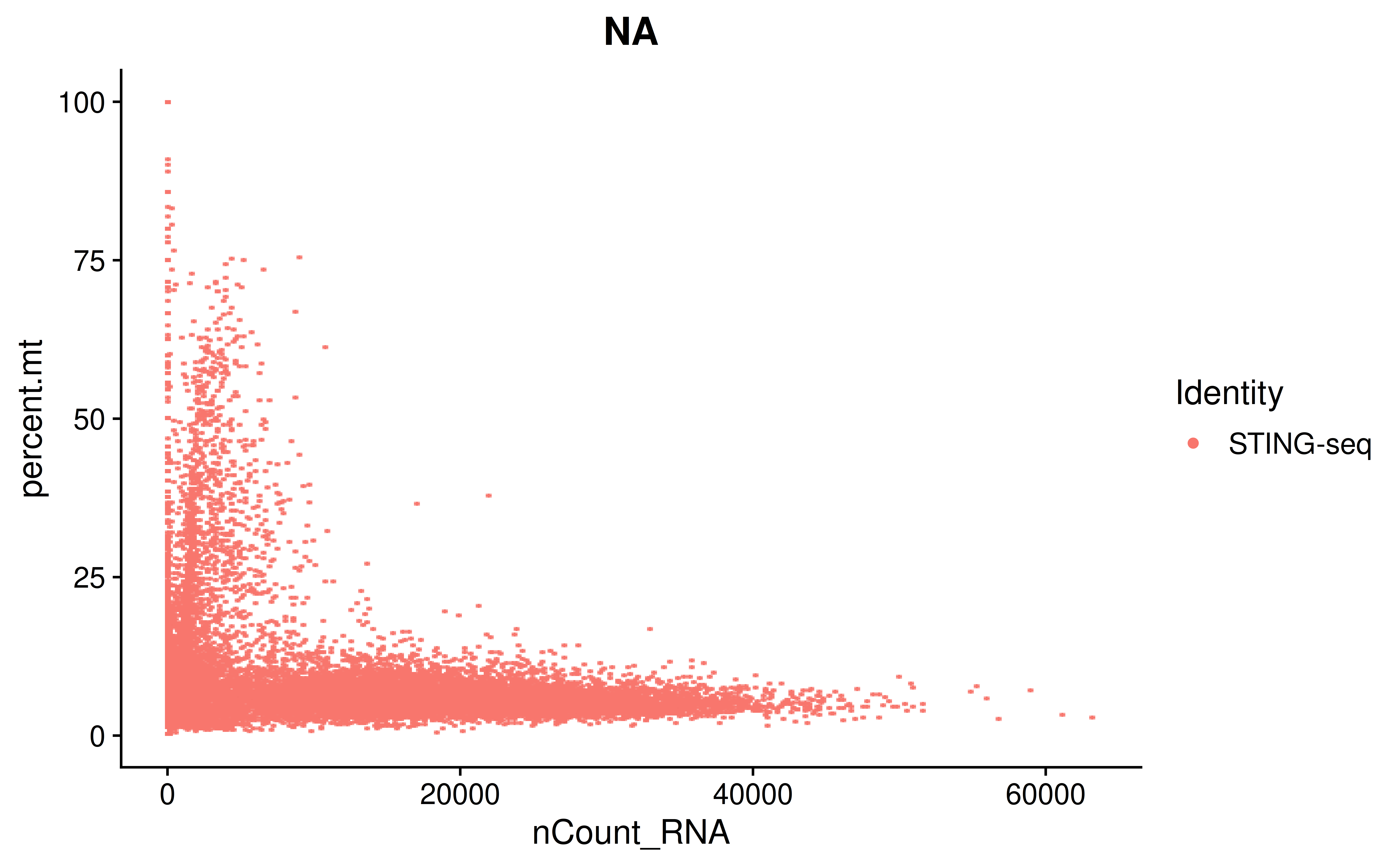
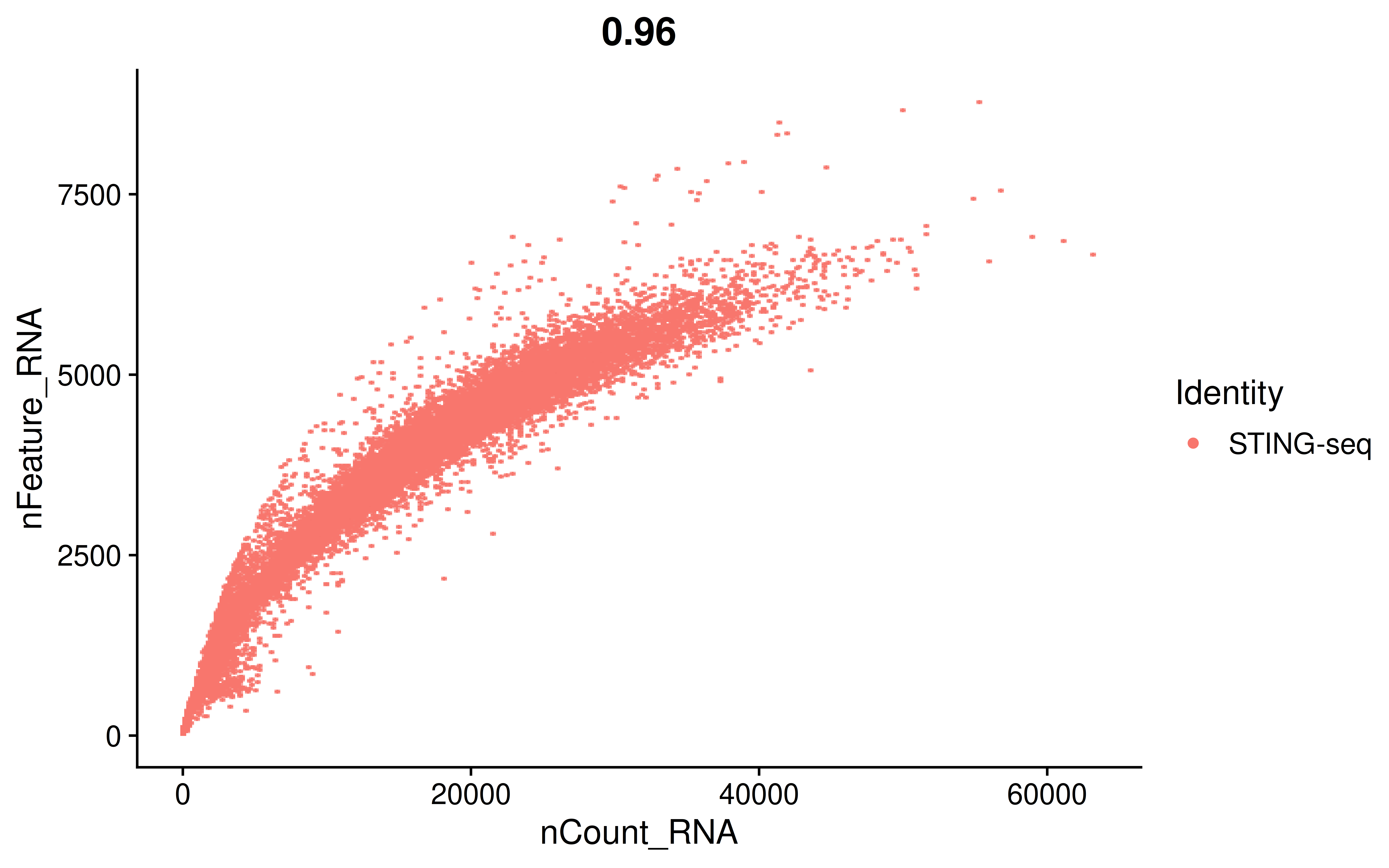
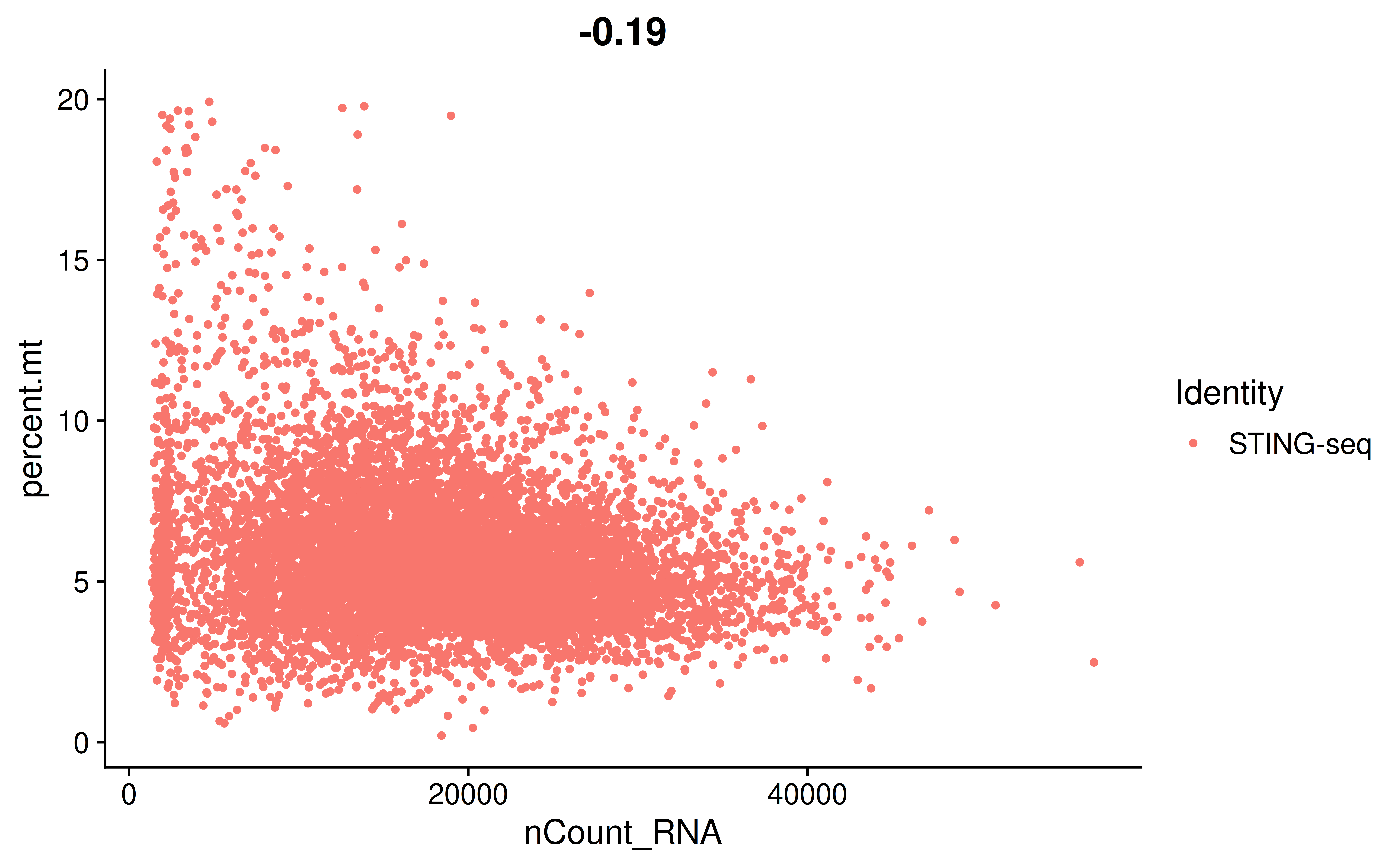
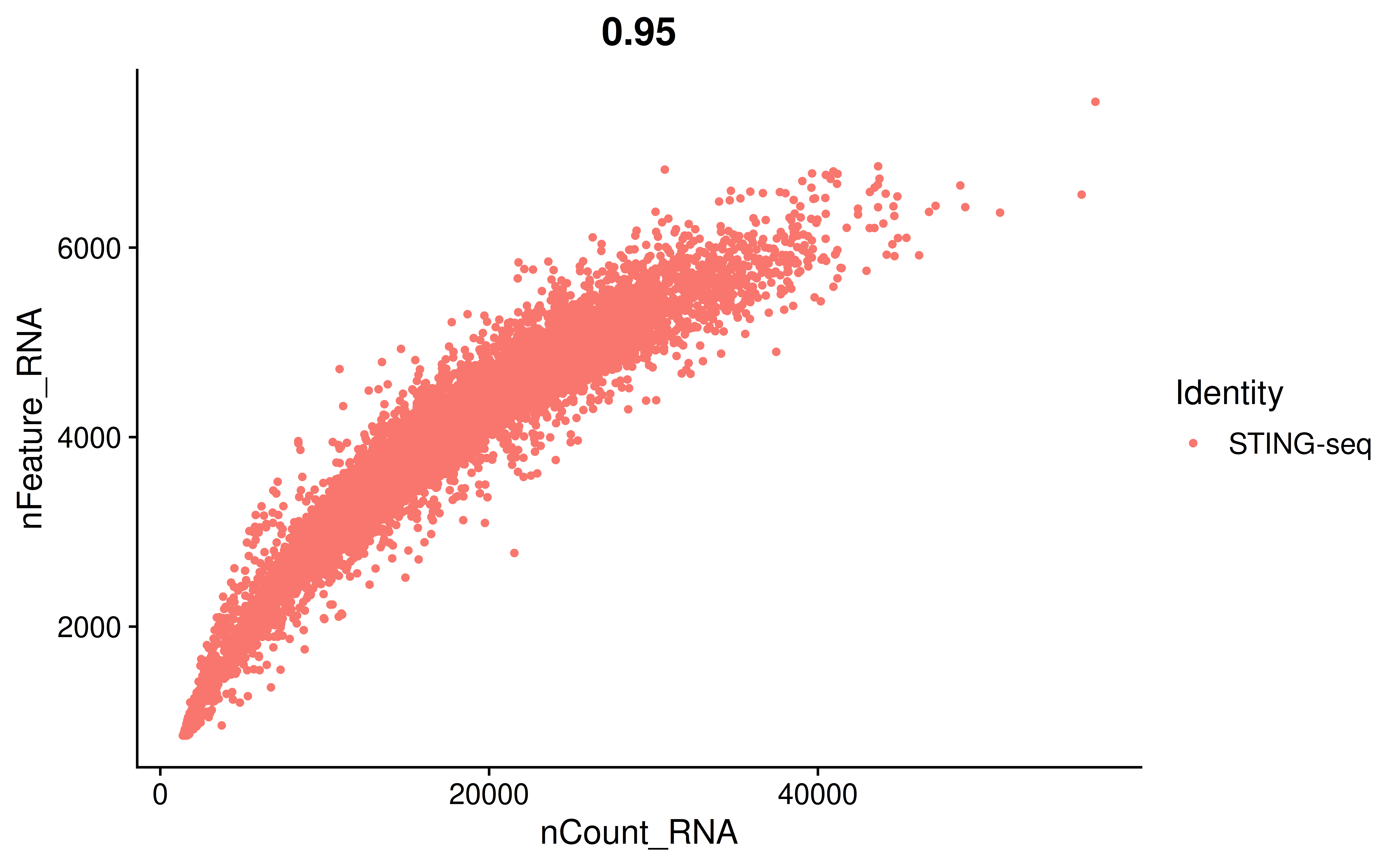
After percent-mito filtering (generated by Seurat):

Barcodes comparison:
Code: QC_compare.R
QC_by_author.txt
QC_by_hang.txt
Comparison result:
[1] "There are 508 cells filtered out in comparison to authors' list."g2. PDF y axis issue
Previous question:
- Is the small values in the Y-axis of the previous PDF plot wrong?
My understanding: probably NOT wrong.Point 1: similar results by a package for epdf
Plotted with base R:
hist(barcode_dist, freq=F, breaks=150, main="PDF: UMI counts in each barcode (cell)", xlab="UMI counts in each barcode (cell)", ylab="PDF")
Plotted with EnvStats package:
EnvStats::epdfPlot(barcode_dist, epdf.col = "red").png)
Point 2: area of the plot is ~1 by eye
Didn’t bother to do calculus, just very roughly calculated
1.5e-04 x 7000 = 1.05
h. zero-inflated plot & regression
Ref: Kim et al. 2020
Code: zero-flated.R
.png)
.png)
regression summary
summary(glm(zero_prop ~ target_mean, family = poisson, data = whichmodel_10)) # poissonCall:
glm(formula = zero_prop ~ target_mean, family = poisson, data = whichmodel_10)
Deviance Residuals:
Min 1Q Median 3Q Max
-0.13432 -0.00250 0.01255 0.01294 1.08289
Coefficients:
Estimate Std. Error z value Pr(>|z|)
(Intercept) -0.012969 0.005915 -2.193 0.0283 *
target_mean -0.672805 0.019706 -34.141 <2e-16 ***
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
(Dispersion parameter for poisson family taken to be 1)
Null deviance: 2256.629 on 35374 degrees of freedom
Residual deviance: 88.113 on 35373 degrees of freedom
AIC: Inf
Number of Fisher Scoring iterations: 5summary(glm.nb(zero_prop ~ target_mean, data = whichmodel_10)) # negative binomialCall:
glm.nb(formula = zero_prop ~ target_mean, data = whichmodel_10,
init.theta = 18539.38634, link = log)
Deviance Residuals:
Min 1Q Median 3Q Max
-0.86791 -0.31620 0.01294 0.06140 1.32706
Coefficients:
Estimate Std. Error z value Pr(>|z|)
(Intercept) -0.012969 0.005915 -2.193 0.0283 *
target_mean -0.672803 0.019707 -34.141 <2e-16 ***
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
(Dispersion parameter for Negative Binomial(18539.39) family taken to be 1)
Null deviance: 6991.6 on 35374 degrees of freedom
Residual deviance: 4823.2 on 35373 degrees of freedom
AIC: 66516
Number of Fisher Scoring iterations: 1
Theta: 18539
Std. Err.: 7723
Warning while fitting theta: iteration limit reached
2 x log-likelihood: -66509.53 i. re-QC
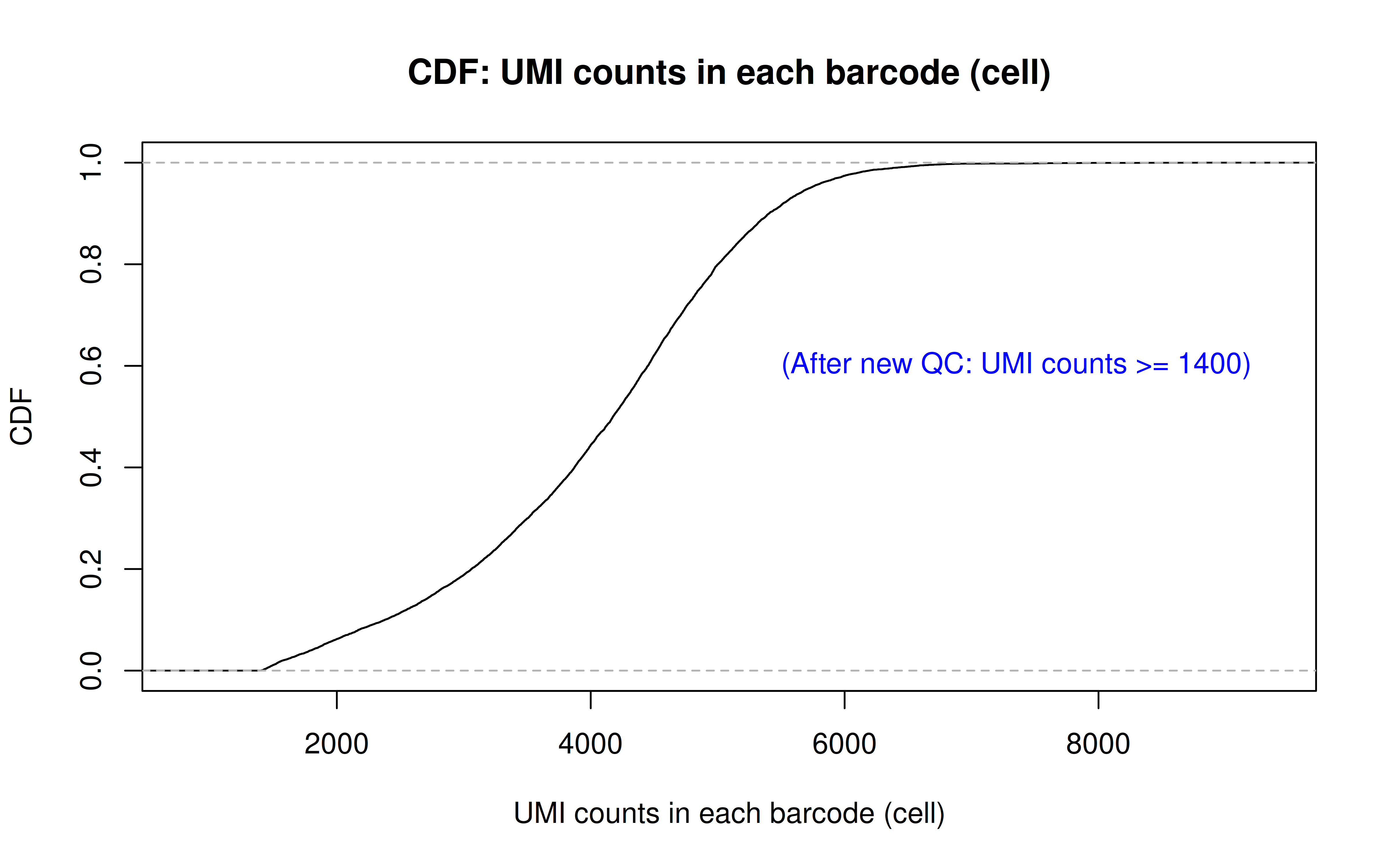
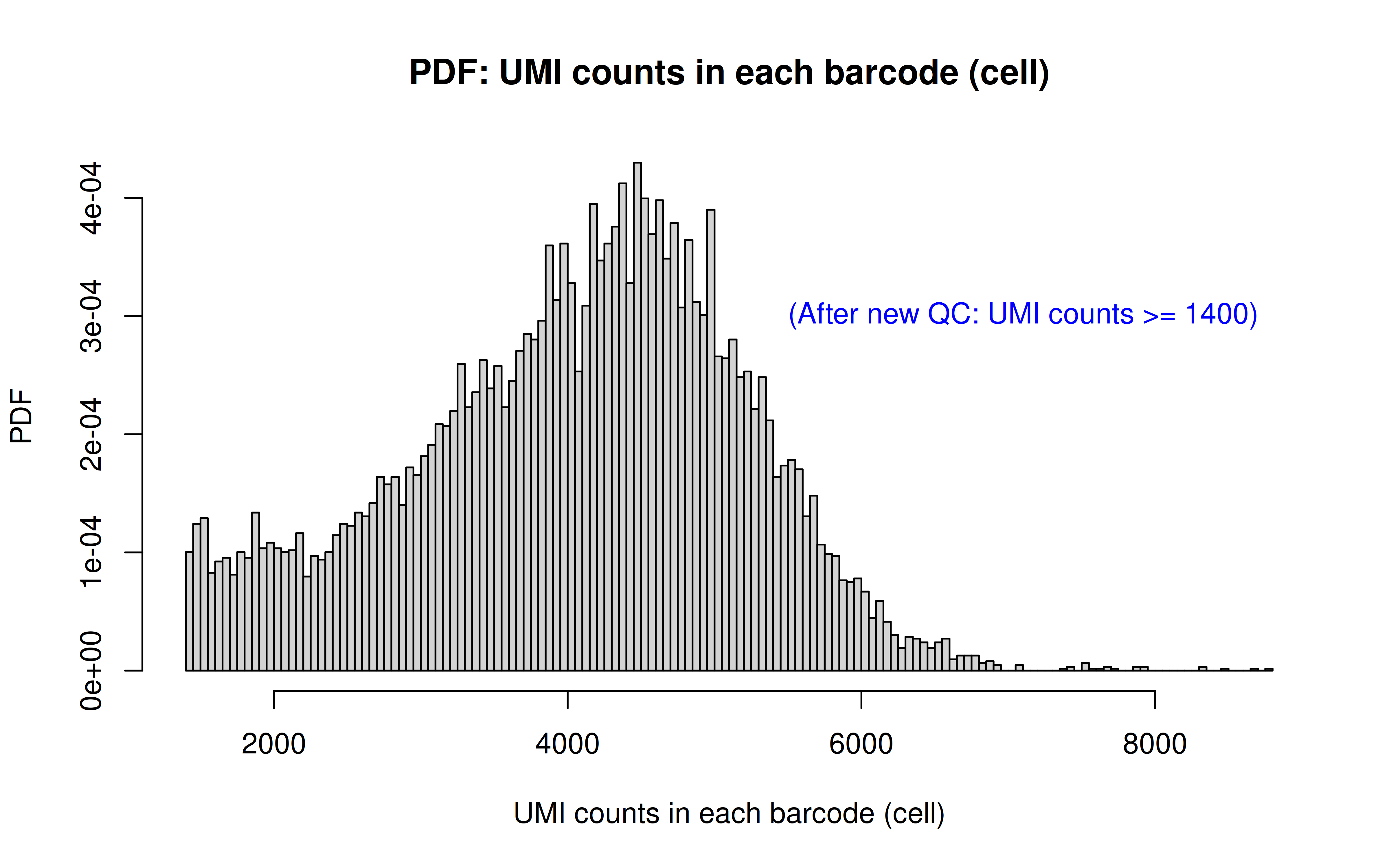
Note: this is to remove the dead cells using a more stringent UMI cutoff (850 originally, 1400 here)
Code: QC_filter_2.R
Code: UMI_plot_2.R
Before UMI count filtering:
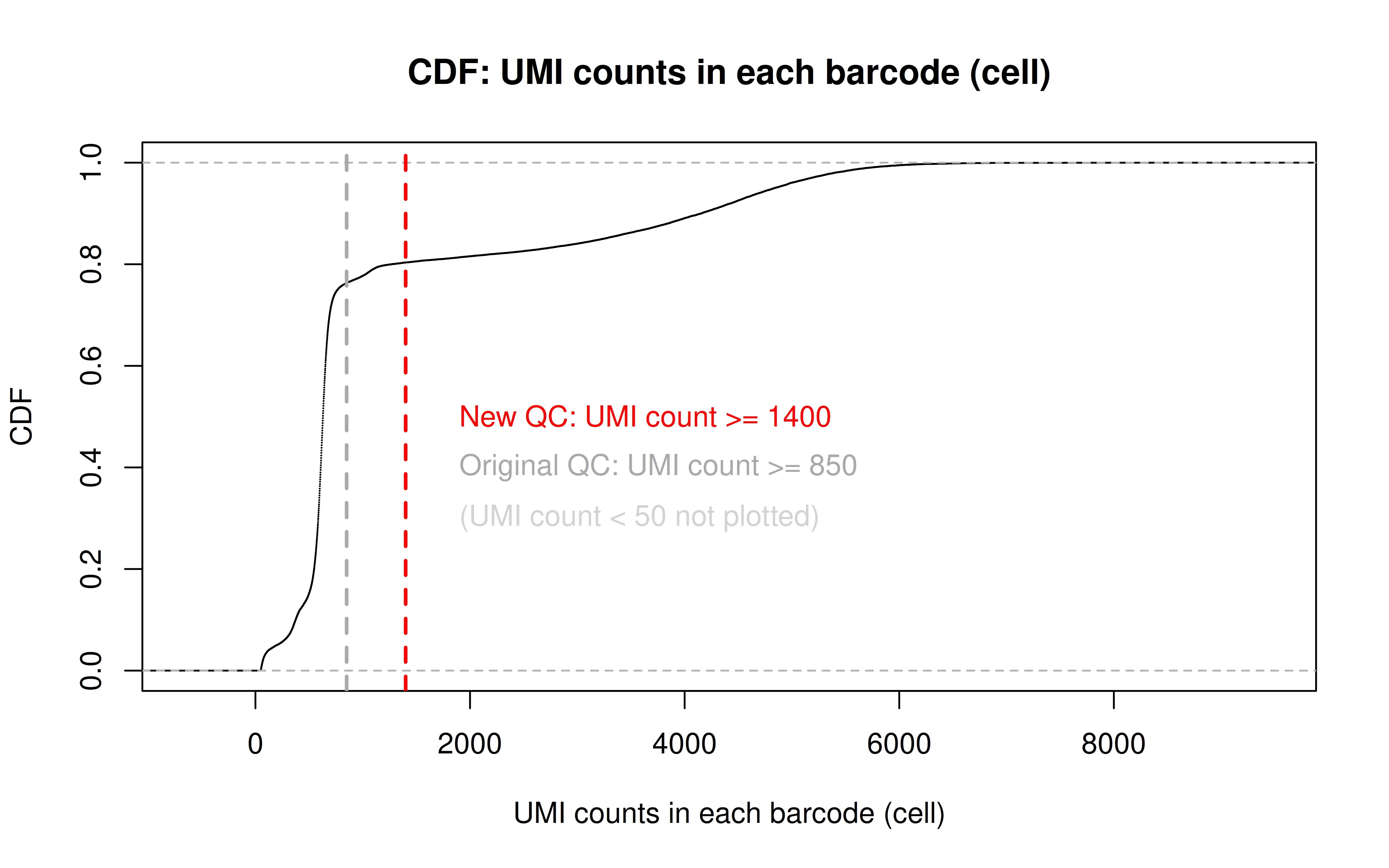
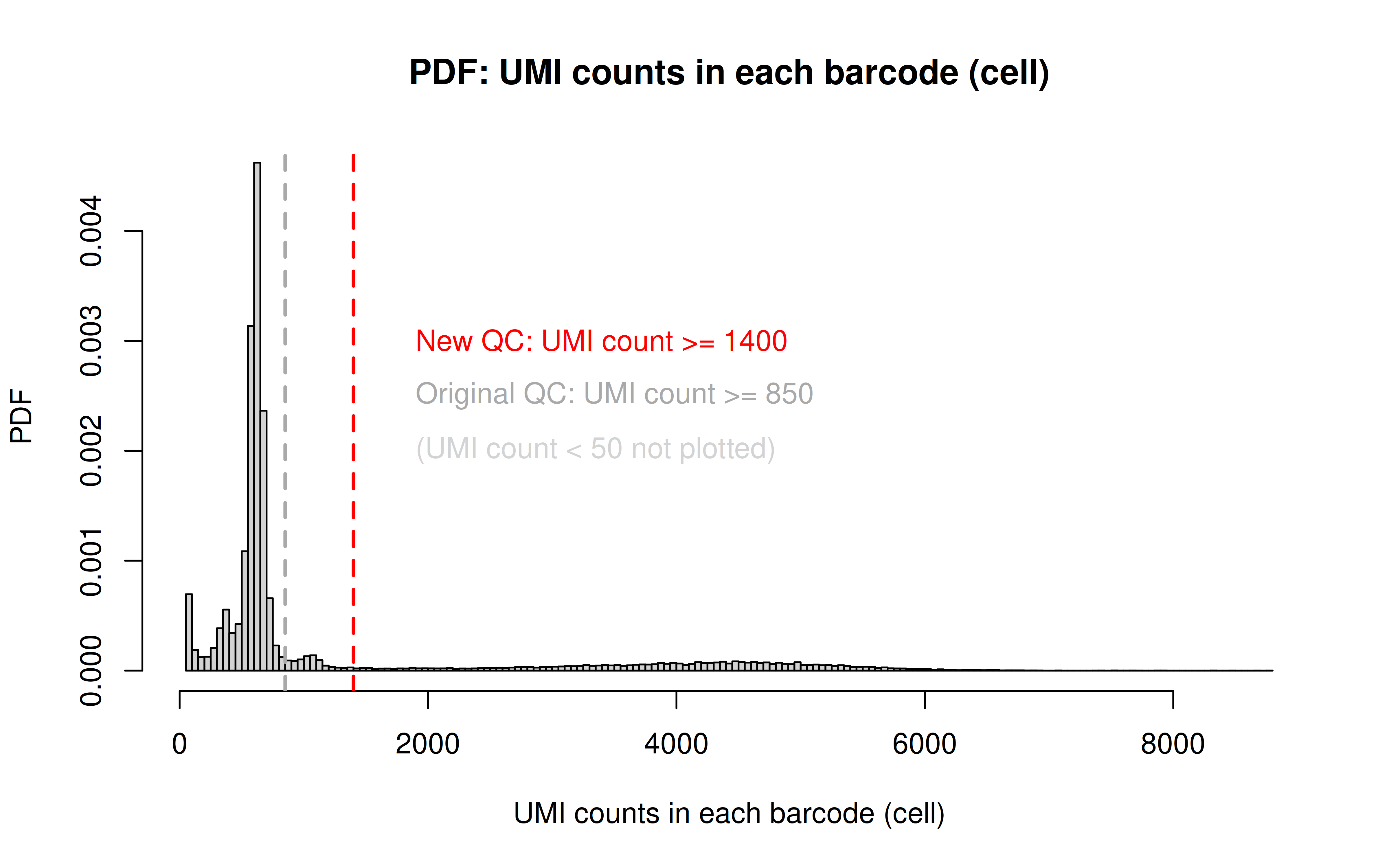
After UMI count filtering:
Before percent-mito filtering (generated by Seurat):
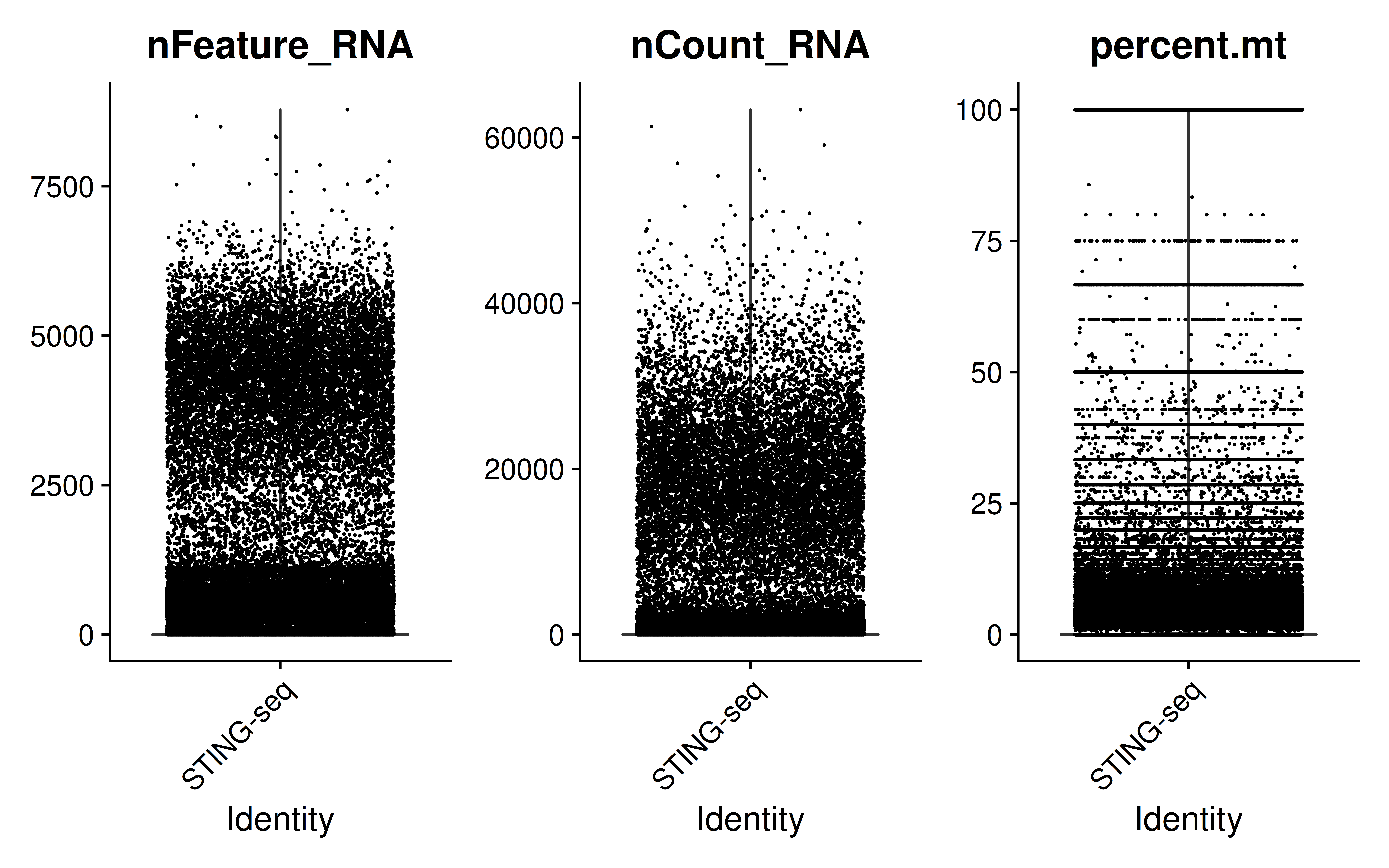
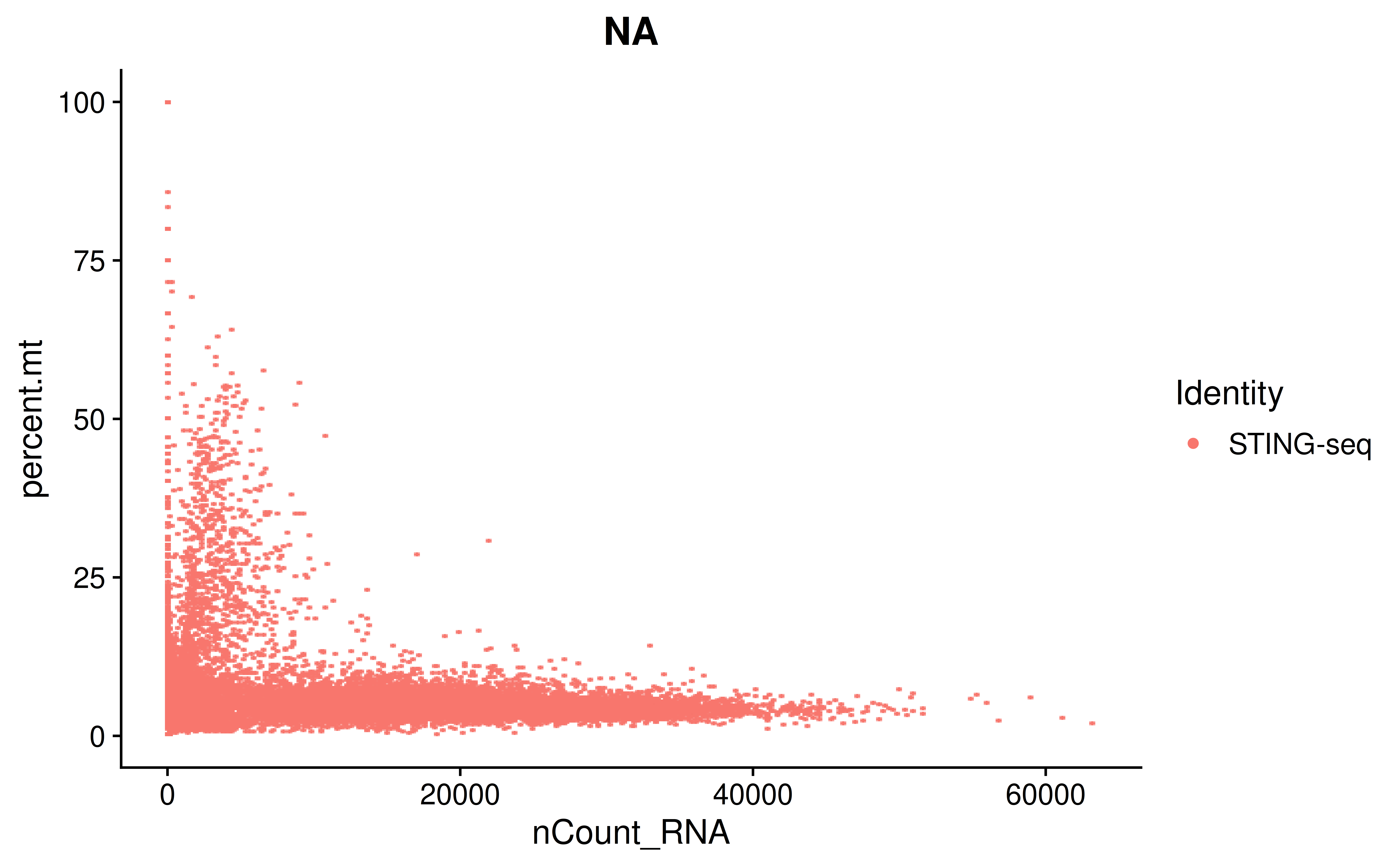
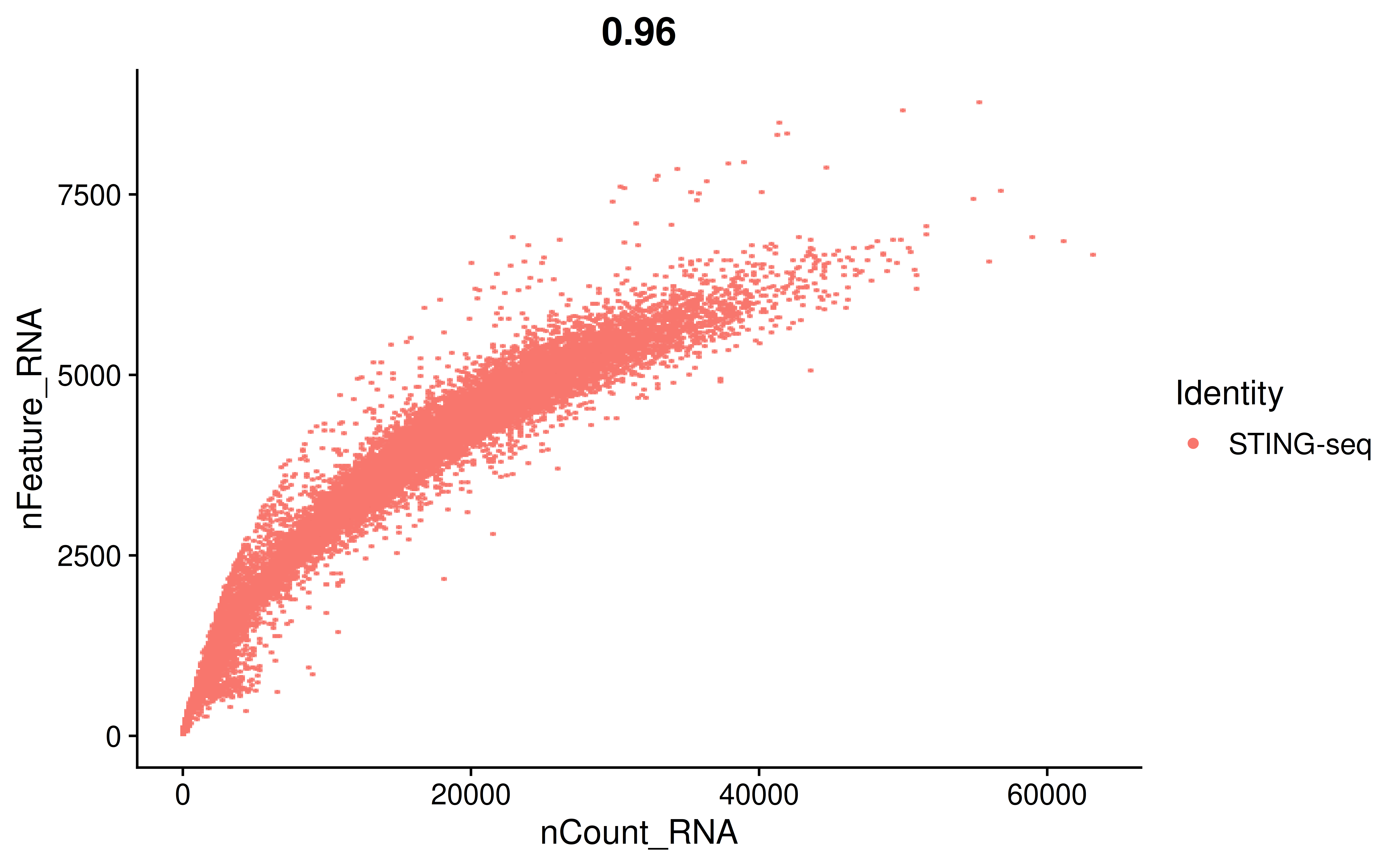
After percent-mito filtering (generated by Seurat):

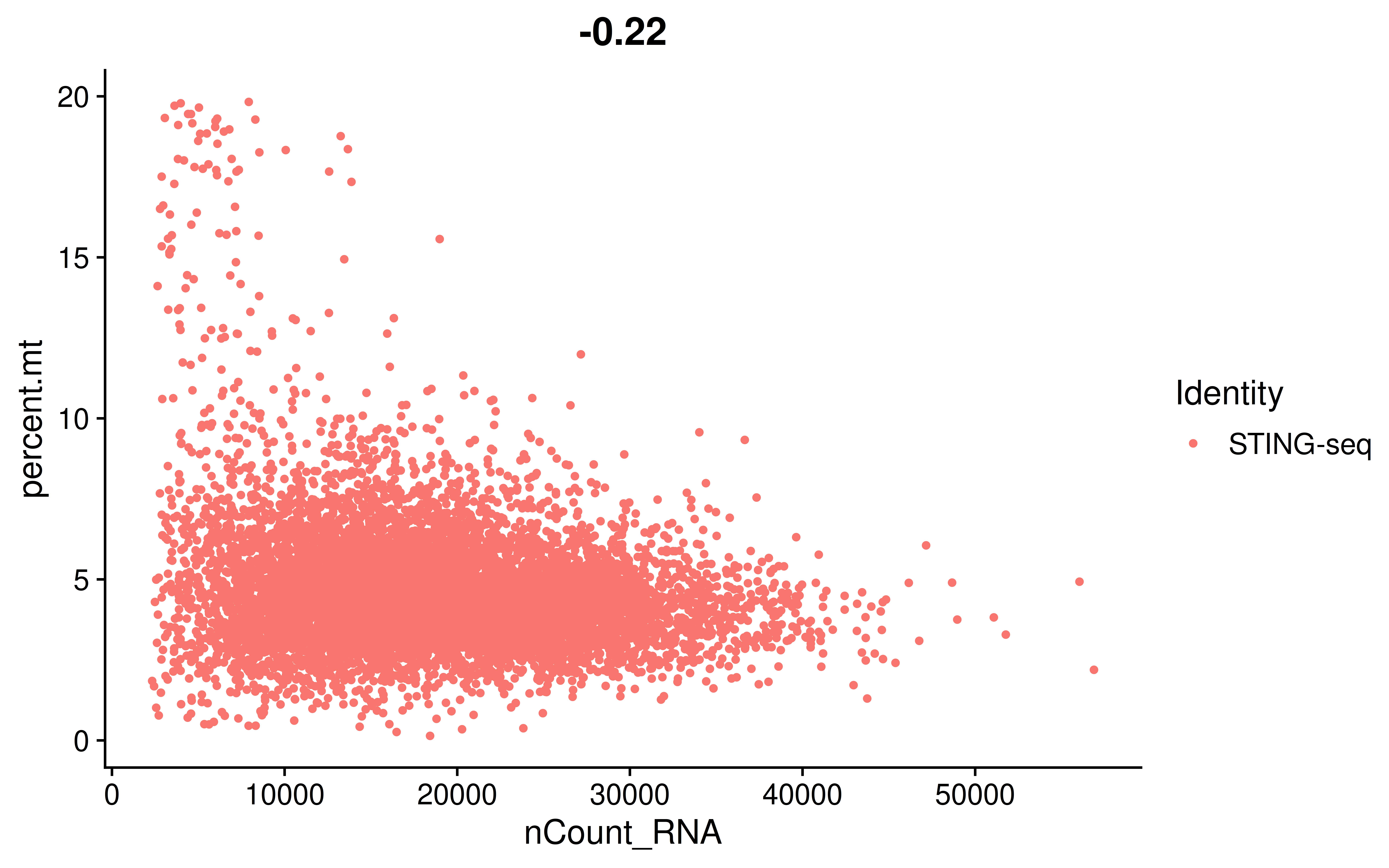
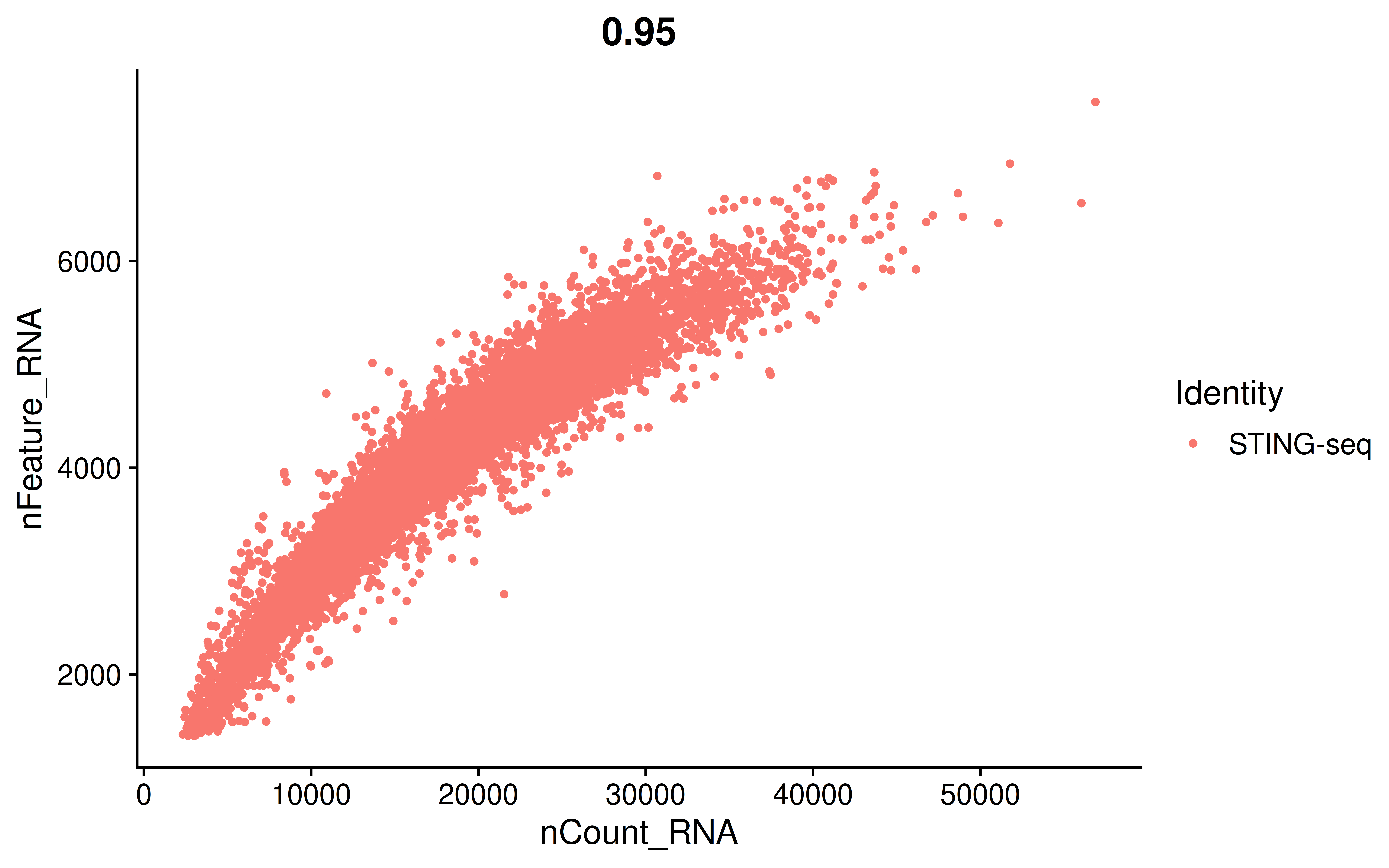
Barcodes comparison:
Code: QC_compare_2.R
QC_by_author.txt
QC_by_hang_2.txt
Comparison result:
[1] "There are 755 cells filtered out in comparison to authors' list."
sessionInfo()R version 4.2.1 (2022-06-23)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Ubuntu 22.04.1 LTS
Matrix products: default
BLAS: /usr/lib/x86_64-linux-gnu/blas/libblas.so.3.10.0
LAPACK: /usr/lib/x86_64-linux-gnu/lapack/liblapack.so.3.10.0
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] workflowr_1.7.0
loaded via a namespace (and not attached):
[1] Rcpp_1.0.9 compiler_4.2.1 pillar_1.8.0 bslib_0.3.1
[5] later_1.3.0 git2r_0.30.1 jquerylib_0.1.4 tools_4.2.1
[9] getPass_0.2-2 digest_0.6.29 jsonlite_1.8.0 evaluate_0.15
[13] tibble_3.1.7 lifecycle_1.0.1 pkgconfig_2.0.3 rlang_1.0.2
[17] cli_3.3.0 rstudioapi_0.13 yaml_2.3.5 xfun_0.31
[21] fastmap_1.1.0 httr_1.4.3 stringr_1.4.0 knitr_1.39
[25] sass_0.4.1 fs_1.5.2 vctrs_0.4.1 rprojroot_2.0.3
[29] glue_1.6.2 R6_2.5.1 processx_3.6.1 fansi_1.0.3
[33] rmarkdown_2.14 callr_3.7.0 magrittr_2.0.3 whisker_0.4
[37] ps_1.7.1 promises_1.2.0.1 htmltools_0.5.2 ellipsis_0.3.2
[41] httpuv_1.6.5 utf8_1.2.2 stringi_1.7.6